|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C22H22IN3O3 |
|||
| Molecular Weight | 503.33 | CAS No. | 444912-48-5 | |
| Solubility (25°C)* | In vitro | DMSO | 101 mg/mL (200.66 mM) | |
| Water | Insoluble | |||
| Ethanol | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | AM-1241 is a selective cannabinoid CB2 receptor agonist with Ki of 3.4 nM, exhibits 82-fold selectivity over CB1 receptor. | ||||
|---|---|---|---|---|---|
| Targets |
|
||||
| In vitro | AM-1241 is a protean agonist of CB2 based on the different effect observed in various assays (calcium influx, extracellular signal-regulated kinase (ERK) phosphorylatin and cAMP measurement)) and on the switch from neutral antagonism to agonism in the cAMP assay when forskolin concentration is lowered. In [3H]CP 55,940 competition binding assays, AM-1241 displays high affinity at the human CB2 receptor with a Ki value of about 7 nM, whereas its affinity at the human CB1 receptor is more than 80-fold weaker, using membrane preparations from stable HEK and CHO cell lines expressing the recombinant human CB2 and CB1 receptors, respectively. [2] | ||||
| In vivo | AM-1241 dose-dependently reverses tactile and thermal hypersensitivity produced by ligation of the L5 and L6 spinal nerves in rats. AM-1241 is also active in blocking spinal nerve ligation-induced tactile and thermal hypersensitivity in mice lacking CB1 receptors (CB1-/- mice), confirming that AM-1241 reverses sensory hypersensitivity independent of actions at CB1 receptors.[1] AM-1241 (100, 330 μg/kg i.p.) suppresses the development of carrageenan-evoked thermal and mechanical hyperalgesia and allodynia. And this suppression is blocked by CB2 antagonist SR144528 but not by CB1 antagonist SR141716A. [3] AM1241 produces dose-dependent antinociception to a thermal stimulus applied to the hindpaw, when administered into the hindpaw on the side of testing (ipsilateral i. paw), while much less active into the contralateral to the side. A50 (analgesic dose yielding a 50% effect) of AM1241 is 847 μg/kg with the maximum possible effect (100% MPE) being achieved at 3.3 mg/kg. AM1241 also produces dose-dependent antinociception when administered intraperitoneally (i.p.), with an A50 of 103μg/kg. The antinociceptive actions of AM1241 are blocked by the CB2 receptor-selective antagonist AM630, but not by the CB1 receptor-selective antagonist AM251. AM1241 dosn't produce the CNS cannabinoid effects of hypothermia, catalepsy, inhibition of activity or impaired ambulation, while this tetrad of effects is produced by the mixed CB1/CB2 receptor agonist WIN55,212-2.[4] Daily injections of AM-1241 through a i.p. route, initiated at symptom onset, increases the survival interval after amyotrophic lateral sclerosis (ALS) onset by 56% in a transgenic mouse model of ALS. [5] |
Protocol (from reference)
| Kinase Assay:[1] |
|
|---|---|
| Cell Assay:[2] |
|
| Animal Study:[1] |
|
References
Customer Product Validation
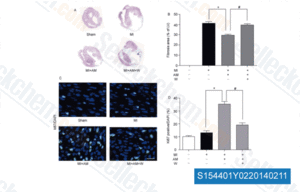
-
Data from [Sci China Life Sci, 2014, 57(2), 201-8]

-
Data from [Data independently produced by , , Cell Physiol Biochem, 2016, 39(4):1521-36]
Selleck's AM1241 has been cited by 16 publications
| CB2R activation ameliorates late adolescent chronic alcohol exposure-induced anxiety-like behaviors during withdrawal by preventing morphological changes and suppressing NLRP3 inflammasome activation in prefrontal cortex microglia in mice [ Brain Behav Immun, 2023, 110:60-79] | PubMed: 36754245 |
| EVs-mediated delivery of CB2 receptor agonist for Alzheimer's disease therapy [ Asian J Pharm Sci, 2023, 18(4):100835] | PubMed: 37645682 |
| EVs-mediated delivery of CB2 receptor agonist for Alzheimer's disease therapy [ Asian J Pharm Sci, 2023, 18(4):100835] | PubMed: 37645682 |
| Activation of cannabinoid receptor 2 attenuates Angiotensin II-induced atrial fibrillation via a potential NOX/CaMKII mechanism [ Front Cardiovasc Med, 2022, 9:968014] | PubMed: 36312282 |
| Fast-Acting and Receptor-Mediated Regulation of Neuronal Signaling Pathways by Copaiba Essential Oil. [ Int J Mol Sci, 2020, 21(7)] | PubMed: 32218156 |
| The fatty-acid amide hydrolase inhibitor URB597 inhibits MICA/B shedding [ Sci Rep, 2020, 10(1):15556] | PubMed: 32968163 |
| Pharmacological activation of CB2 receptor protects against ethanol-induced myocardial injury related to RIP1/RIP3/MLKL-mediated necroptosis [ Mol Cell Biochem, 2020, 10.1007/s11010-020-03828-1] | PubMed: 32681290 |
| Crocetin Alleviates Inflammation in MPTP-Induced Parkinson's Disease Models through Improving Mitochondrial Functions [ Parkinsons Dis, 2020, 2020:9864370] | PubMed: 33101635 |
| Activation of cannabinoid type 2 receptor protects skeletal muscle from ischemia-reperfusion injury partly via Nrf2 signaling. [ Life Sci, 2019, 230:55-67] | PubMed: 31128135 |
| Pretreatment with AM1241 Enhances the Analgesic Effect of Intrathecally Administrated Mesenchymal Stem Cells. [ Stem Cells Int, 2019, 2019:7025473] | PubMed: 31611918 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
