|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C18H17FN4O |
|||
| Molecular Weight | 324.35 | CAS No. | 934162-61-5 | |
| Solubility (25°C)* | In vitro | DMSO | 65 mg/mL (200.4 mM) | |
| Water | Insoluble | |||
| Ethanol | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | A-966492 is a novel and potent inhibitor of PARP1 and PARP2 with Ki of 1 nM and 1.5 nM, respectively. | ||||||
|---|---|---|---|---|---|---|---|
| Targets |
|
||||||
| In vitro | A-966492 is one of the most potent PARP inhibitors. A-966492 displays excellent potency against the PARP-1 enzyme with a Kiof 1 nM and an EC50 of 1 nM in a whole cell assay. A-966492 significantly enhances the efficacy of TMZ in a dose-dependent manner. In addition, A-966492 is orally bioavailable across multiple species, crosses the blood−brain barrier, and appears to distribute into tumor tissue. A-966492 represents a promising, structurally diverse benzimidazole analogue and is being further characterized preclinically. [1] | ||||||
| In vivo | A-966492 also demonstrates good in vivo efficacy in a B16F10 subcutaneous murine melanoma model in combination with temozolomide and in an MX-1 breast cancer xenograft model both as a single agent and in combination with carboplatin. In addition, A-966492 has excellent pharmaceutical properties and has demonstrated in vivo efficacy in preclinical mouse tumor models in combination with TMZ and carboplatin, as well as single agent activity in a BRCA1-deficient MX-1 tumor model. A-966492 is further characterized in Sprague−Dawley rats, beagle dogs, and cynomolgus monkeys, with A-966492 demonstrating oral bioavailabilities of 34−72% and half-lives of 1.7−1.9 hours. In vivo, A-966492 demonstrates significant enhancement of the efficacy of TMZ in a murine B16F10 syngeneic melanoma model, with the A-966492 combination groups showing superior efficacy. [1] | ||||||
| Features | A promising, structurally diverse benzimidazole analogue that is being further characterized preclinically. |
Protocol (from reference)
| Kinase Assay: |
|
|---|---|
| Cell Assay: |
|
| Animal Study: |
|
Customer Product Validation
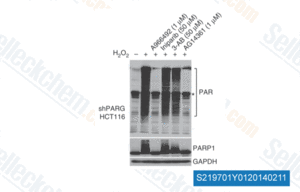
-
Data from [Nat Methods , 2013, 10(10), 981-4]
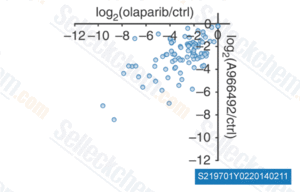
-
Data from [Nat Methods , 2013, 10(10), 981-4]
Selleck's A-966492 has been cited by 11 publications
| Histone Parylation factor 1 contributes to the inhibition of PARP1 by cancer drugs [ Nat Commun, 2021, 12(1):736] | PubMed: 33531508 |
| Pharmacological Poly (ADP-Ribose) Polymerase Inhibitors Decrease Mycobacterium tuberculosis Survival in Human Macrophages [ Front Immunol, 2021, 12:712021] | PubMed: 34899683 |
| Pharmacological Poly (ADP-Ribose) Polymerase Inhibitors Decrease Mycobacterium tuberculosis Survival in Human Macrophages [ Front Immunol, 2021, 12:712021] | PubMed: 34899683 |
| CRISPR screening identifies novel PARP inhibitor classification based on distinct base excision repair pathway dependencies [ bioRxiv, 2021, 10.1101/2020.10.18.333070] | PubMed: None |
| Response of Breast Cancer Cells to PARP Inhibitors Is Independent of BRCA Status. [ J Clin Med, 2020, 30;9(4)] | PubMed: 32235451 |
| [ Cancer Immunol Res, 2019, ] | PubMed: 30401677 |
| Short half-life of HPV16 E6 and E7 mRNAs sensitizes HPV16-positive tonsillar cancer cell line HN26 to DNA-damaging drugs [Wu C Int J Cance, 2019, 144(2):297-310] | PubMed: 30303514 |
| The Toxmatrix: Chemo-Genomic Profiling Identifies Interactions That Reveal Mechanisms of Toxicity [ Chem Res Toxicol, 2018, 31(2):127-136] | PubMed: 29156121 |
| The Toxmatrix: Chemo-Genomic Profiling Identifies Interactions That Reveal Mechanisms of Toxicity [ Chem Res Toxicol, 2018, 31(2):127-136] | PubMed: 29156121 |
| The combination of A-966492 and Topotecan for effective radiosensitization on glioblastoma spheroids [Koosha F Biochem Biophys Res Commun, 2017, 491(4):1092-1097] | PubMed: 28797568 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
