|
How to Cite 1. For In-Text Citation (Materials & Methods): 2. For Key Resources Table: |
||
|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. We strive to reply to |
Technical Data
| Formula | C17H14BrN3O |
||||||
| Molecular Weight | 356.22 | CAS No. | 857064-38-1 | ||||
| Solubility (25°C)* | In vitro | DMSO | 71 mg/mL (199.31 mM) | ||||
| Water | Insoluble | ||||||
| Ethanol | Insoluble | ||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||
Preparing Stock Solutions
Biological Activity
| Description | WP1066 is a novel inhibitor of JAK2 and STAT3 with IC50 of 2.30 μM and 2.43 μM in HEL cells; shows activity to JAK2, STAT3, STAT5, and ERK1/2 not JAK1 and JAK3. WP1066 induces apoptosis. Phase 1. | ||||
|---|---|---|---|---|---|
| Targets |
|
||||
| In vitro | WP1066 markedly inhibits the growth of HEL cells carrying the JAK2 V617F mutant isoform in a dose-dependent manner with IC20, IC50 and IC80 of 0.8, 2.3 and 3.8 μM. This compound at concentrations of 0.5, 1.0, 2.0, 3.0, or 4.0 μM inhibits the phosphorylation of JAK2, STAT3, STAT5, and ERK1/2 without affecting the phosphorylation of JAK1 and JAK3 in erythroid leukemia HEL cells that express the JAK2 V617F isoform. It at concentrations ranging from 0.5 to 3.0 μM inhibits the proliferation of AML colony-forming cells obtained from patients and that of the AML cell lines OCIM2 and K562 in a dose-dependent manner. This chemical at concentrations of 0.5, 1.0, 2.0, 3.0, or 4.0 μM dose-dependently decreases JAK2 and pJAK2 protein levels as well as downstream phosphorylation levels of STAT3, STAT5, and AKT in OCIM2 and K562 cells. It at concentrations of 2 μM inhibits OCIM2 cell multiplication by inducing accumulation of cells at the G0-G1 phase of the cell cycle. This compound at concentrations of 1, 2, or 3 μM induces apoptosis in both OCIM2 and K562 cells in a dose-dependent fashion by activating procaspase-3 and cleaving PARP. It at concentrations of 5 μM prevents the phosphorylation of STAT3, and at concentrations of 2.5μM it significantly inhibits cell survival and proliferation in Caki-1 and 786-O renal cancer cells. This chemical at concentrations of 5 μM suppresses HIF1α and HIF2α expression and VEGF production in Caki-1 and 786-O renal cancer cells. | ||||
| In vivo | WP1066 orally administrated at dose of 40 mg/kg once daily for 19 days significantly inhibits the tumours growth in Caki-1 xenograft mice, with decreased immunostaining of phosphorylated STAT3 and reduced length of CD34-positive vessels. | ||||
| Features | Similar to its parent compound AG490, WP1066 inhibits the phosphorylation of JAK2, but unlike AG490, WP1066 also degraded JAK2 protein. |
Protocol (from reference)
| Cell Assay:[1] |
|
|---|---|
| Animal Study:[4] |
|
References
|
Customer Product Validation
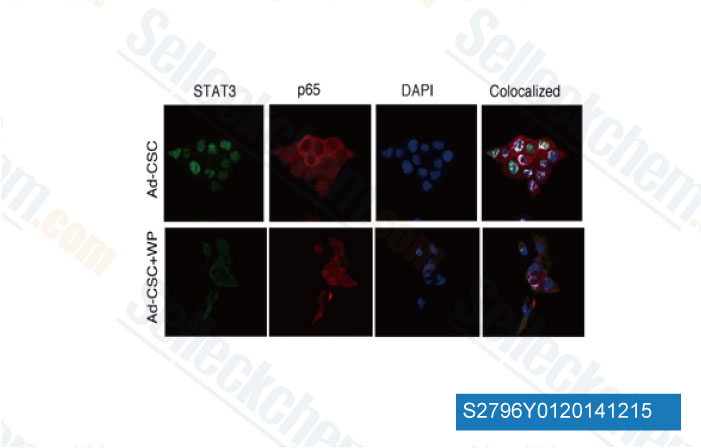
-
Data from [ J Biol Chem , 2013 , 288(36), 26167-76 ]
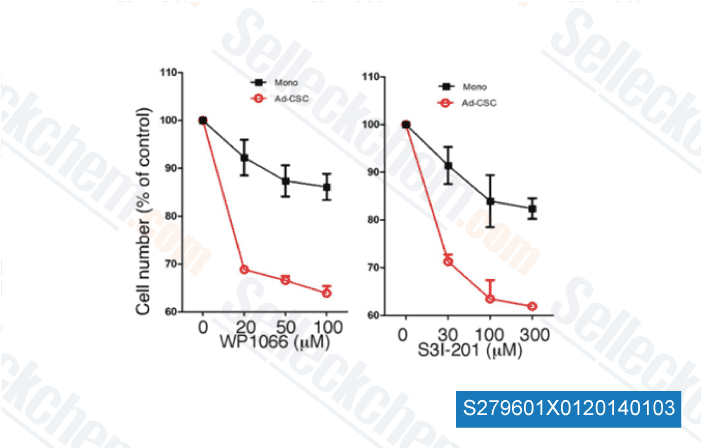
-
,
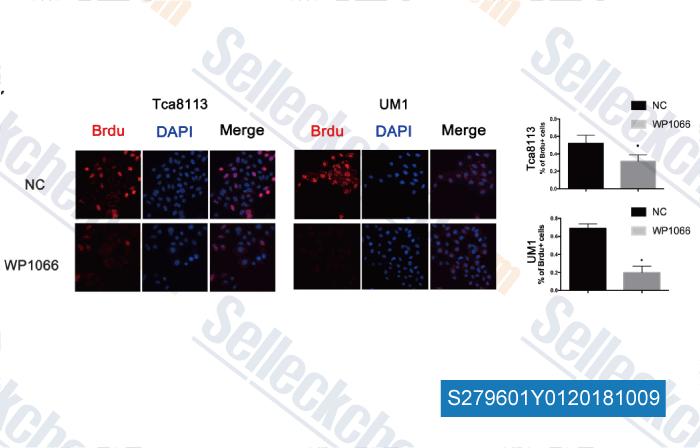
-
Data from [ , , Clin Cancer Res, 2018, 24(11):2665-2677 ]
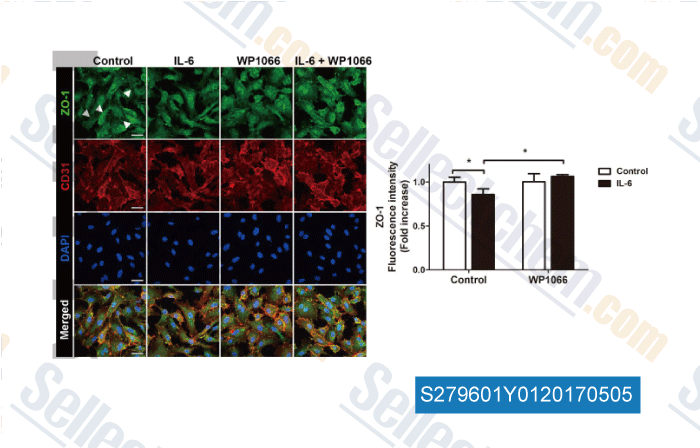
-
Data from [ , , J Cell Physiol, 2017, 232(5):1123-1134 ]
Selleck's WP1066 Has Been Cited by 76 Publications
| NIBAN2 Stimulates Glioma Growth by Regulating the JAK2/STAT3/c-Myc Pathway [ Cancer Med, 2025, 14(18):e71239] | PubMed: 40944417 |
| Lactate dehydrogenase A regulates tumor-macrophage symbiosis to promote glioblastoma progression [ Nat Commun, 2024, 15(1):1987] | PubMed: 38443336 |
| IGFBP3 induces PD-L1 expression to promote glioblastoma immune evasion [ Cancer Cell Int, 2024, 24(1):60] | PubMed: 38326861 |
| RNF122 promotes glioblastoma growth via the JAK2/STAT3/c-Myc signaling Axis [ CNS Neurosci Ther, 2024, 30(9):e70017] | PubMed: 39218810 |
| Investigation of cuproptosis regulator-mediated modification patterns and SLC30A7 function in GBM [ Aging (Albany NY), 2024, 16(4):3554-3582] | PubMed: 38393693 |
| P. gingivalis Infection Upregulates PD-L1 Expression on Dendritic Cells, Suppresses CD8+ T-cell Responses, and Aggravates Oral Cancer [ Cancer Immunol Res, 2023, 11(3):290-305] | PubMed: 36633576 |
| Tumor-associated astrocytes promote tumor progression of Sonic Hedgehog medulloblastoma by secreting lipocalin-2 [ Brain Pathol, 2023, 10.1111/bpa.13212] | PubMed: 37721122 |
| oHSV-P10 reduces glioma stem cell enrichment after oncolytic HSV therapy [ Mol Ther Oncolytics, 2023, 29:30-41] | PubMed: 37114074 |
| Triptolide reduces PD-L1 through the EGFR and IFN-γ/IRF1 dual signaling pathways [ Int Immunopharmacol, 2023, 118:109993] | PubMed: 36931170 |
| Triptolide reduces PD-L1 through the EGFR and IFN-γ/IRF1 dual signaling pathways [ International Immunopharmacology, 2023, Volume 118] | PubMed: None |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
