|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C24H25N5O3S |
||||||
| Molecular Weight | 463.55 | CAS No. | 1232416-25-9 | ||||
| Solubility (25°C)* | In vitro | DMSO | 24 mg/mL (51.77 mM) | ||||
| Water | Insoluble | ||||||
| Ethanol | Insoluble | ||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||
Preparing Stock Solutions
Biological Activity
| Description | Berzosertib (VE-822, VX970, M6620) is an ATR inhibitor with IC50 of 19 nM in HT29 cells. | ||
|---|---|---|---|
| Targets |
|
||
| In vitro | VE-822 (80 nM) attenuates ATR signaling pathway and reduces survival in tumor cells in response to XRT and LY-188011. VE-822 (80 nM) attenuates ATR signaling in normal cells without enhancing radiation and LY-188011 killing in normal cells. VE-822 (80 nM) increases XRT-induced residual γH2AX and 53BP1 foci compared with XRT in MiaPaCa-2 and PSN-1 cells. VE-822 (80 nM) pre-treatment decreases Rad51 foci after XRT in MiaPaCa-2 and PSN-1 cells. VE-822 (80 nM) alone increases the G1-phase-fraction in MiaPaCa-2 and PSN-1 cells. VE-822 (80 nM) abrogates XRT enriched G2/M-phase-fraction in MiaPaCa-2 and PSN-1 cells. VE-822 has little effect alone, while VE-822 (80 nM) combined with XRT and/or LY-188011 enhances early and late apoptosis in PSN-1 cells that is strongest in the triple combination. [1] VE-822 increases tumor response to DNA damaging agents associated with blockade of pChk1 Ser345. [2] |
||
| In vivo | VE-822 (60 mg/kg) inhibits phospho-Ser-345-Chk1 in mice bearing PSN-1 tumors after DNA-damaging agents. VE-822 (60 mg/kg) combined with XTR doubles the time for tumors to grow to 600 mm3 of XRT alone in mice bearing both PSN-1 and MiaPaCa-2 tumors. VE-822 (60 mg/kg) added to the combination of LY-188011 and XRT substantially prolongs the tumor growth delay compared with the Gem+XRT1 group n mice bearing both PSN-1 tumors. VE-822 (60 mg/kg) combined with XRT1 increases uptake in tumors by 44% compared with XRT1, suggesting that addition of VE-822 increased γH2AX phosphorylation and persistence of DNA damage caused by XRT. [1] |
||
| Features | The first ATR-targeted drug candidate with high selectivity for ATR. |
Protocol (from reference)
| Cell Assay: |
|
|---|---|
| Animal Study: |
|
References
|
Customer Product Validation
-
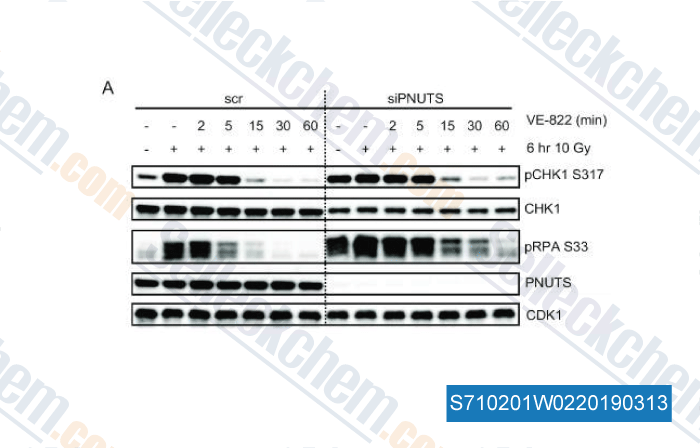
Data from [ , , Nucleic Acids Res, 2018, doi:10.1093/nar/gky1233 ]
-
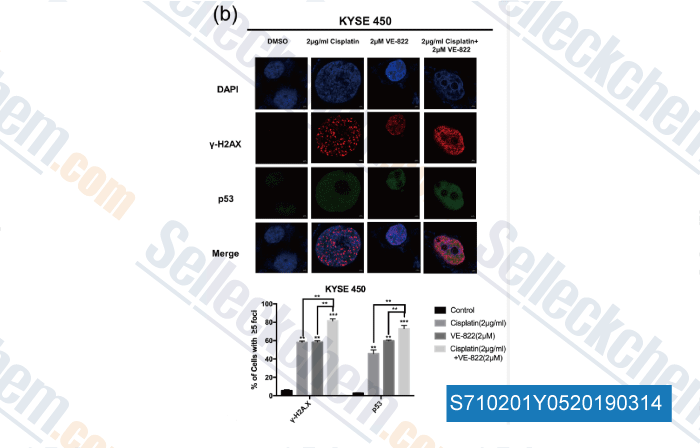
Data from [ , , Cancer Lett, 2018, 432:56-68 ]
-
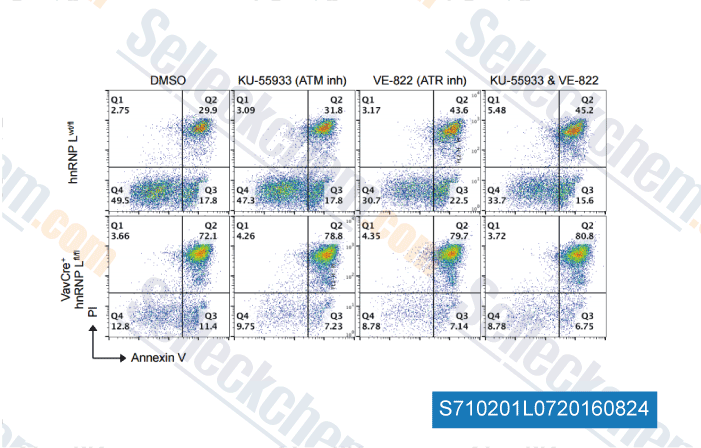
Data from [ , , Sci Rep, 2016, 6:27379. ]
-
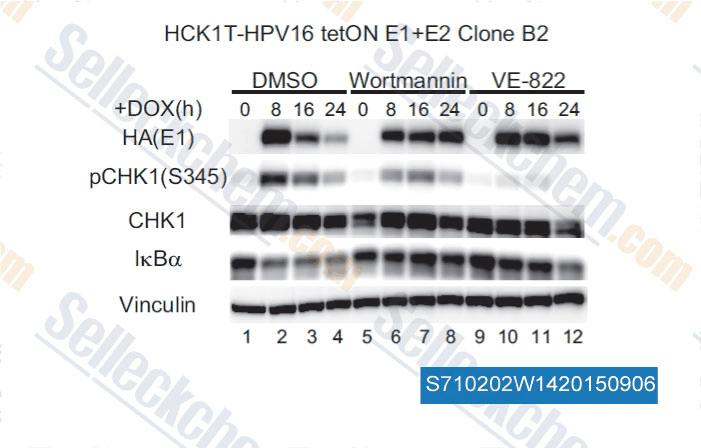
Data from [ , , J Virol, 2015, 89(9): 5040-59 ]
Selleck's Berzosertib (VE-822) has been cited by 147 publications
| KEAP1 and STK11/LKB1 alterations enhance vulnerability to ATR inhibition in KRAS mutant non-small cell lung cancer [ Cancer Cell, 2025, 43(8):1530-1548.e9] | PubMed: 40645185 |
| Homologous recombination promotes non-immunogenic mitotic cell death upon DNA damage [ Nat Cell Biol, 2025, 27(1):59-72] | PubMed: 39805921 |
| Mouse MRE11-RAD50-NBS1 is needed to start and extend meiotic DNA end resection [ Nat Commun, 2025, 16(1):3613] | PubMed: 40240347 |
| Selective targeting of genome amplifications and repeat elements by CRISPR-Cas9 nickases to promote cancer cell death [ Nat Commun, 2025, 16(1):5126] | PubMed: 40456709 |
| The DNA replication checkpoint prevents PCNA/RFC depletion to protect forks from HLTF-induced collapse in human cells [ Mol Cell, 2025, 85(13):2474-2486.e6] | PubMed: 40578346 |
| Gemcitabine and ATR inhibitors synergize to kill PDAC cells by blocking DNA damage response [ Mol Syst Biol, 2025, 21(3):231-253] | PubMed: 39838187 |
| TOX High-Mobility Group Box Family Member 4 promotes DNA double-strand break repair via nonhomologous end joining [ J Biol Chem, 2025, 301(6):110174] | PubMed: 40328361 |
| TEX264 drives selective autophagy of DNA lesions to promote DNA repair and cell survival [ Cell, 2024, 187(20):5698-5718.e26] | PubMed: 39265577 |
| Distinct regulation of ATM signaling by DNA single-strand breaks and APE1 [ Nat Commun, 2024, 15(1):6517] | PubMed: 39112456 |
| Radiotherapy-resistant prostate cancer cells escape immune checkpoint blockade through the senescence-related ataxia telangiectasia and Rad3-related protein [ Cancer Commun (Lond), 2024, 10.1002/cac2.12636] | PubMed: 39698847 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
