|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C21H16ClF3N4O3 |
||||||
| Molecular Weight | 464.82 | CAS No. | 284461-73-0 | ||||
| Solubility (25°C)* | In vitro | DMSO | 92 mg/mL (197.92 mM) | ||||
| Water | Insoluble | ||||||
| Ethanol | Insoluble | ||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||
Preparing Stock Solutions
Biological Activity
| Description | Sorafenib is a multikinase inhibitor of Raf-1 and B-Raf with IC50 of 6 nM and 22 nM in cell-free assays, respectively. Sorafenib inhibits VEGFR-2, VEGFR-3, PDGFR-β, Flt-3 and c-KIT with IC50 of 90 nM, 20 nM, 57 nM, 59 nM and 68 nM, respectively. Sorafenib induces autophagy and apoptosis and activates ferroptosis with anti-tumor activity. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Targets |
|
|||||||||||
| In vitro | Sorafenib inhibits both wild-type and V599E mutant B-Raf activity with IC50 of 22 nM and 38 nM, respectively. Sorafenib also potently inhibits mVEGFR2 (Flk-1), mVEGFR3, mPDGFRβ, Flt3, and c-Kit with IC50 of 15 nM, 20 nM, 57 nM, 58 nM, and 68 nM, respectively. Sorafenib weakly inhibits FGFR-1 with IC50 of 580 nM. Sorafenib tosylate is not active against ERK-1, MEK-1, EGFR, HER-2, IGFR-1, c-Met, PKB, PKA, cdk1/cyclinB, PKCα, PKCγ, and pim-1. Sorafenib markedly inhibits VEGFR2 phosphorylation in NIH 3T3 cells with IC50 of 30 nM, and Flt-3 phosphorylation in HEK-293 cells with IC50 of 20 nM. Sorafenib potently blocks MEK 1/2 and ERK 1/2 phosphorylation in most cell lines but not in A549 or H460 cells, while having no effect on inhibition of the PKB pathway. Sorafenib inhibits the proliferation of HAoSMC and MDA-MB-231 cells with IC50 of 0.28 μM and 2.6 μM, respectively. [1] In addition to inhibition of the RAF/MEK/ERK signaling pathway, Sorafenib significantly inhibits the phosphorylation of eIF4E and down-regulates Mcl-1 levels in hepatocellular carcinoma (HCC) cells in a MEK/ERK-independent manner. Sorafenib inhibits the proliferation of PLC/PRF/5 and HepG2 cells with IC50 of 6.3 μM and 4.5 μM, respectively, and leads to the significant induction of apoptosis. [2] | |||||||||||
| In vivo | Oral administration of Sorafenib (~60 mg/kg) demonstrates broad spectrum, dose-dependent anti-tumor activity against a variety of human tumor xenograft models including MDA-MB-231, Colo-205, HT-29, DLD-1, NCI-H460, and A549, with no evidence of toxicity. In association with the anti-tumor efficacy, Sorafenib treatment potently inhibits MEK 1/2 phosphorylation and pERK 1/2 levels in HT-29 and MDA-MB-231 xenografts but not in Colo-205 xenografts, and significantly suppresses tumor microvessel area (MVA) and microvessel density (MVD) in MDA MB-231, HT-29 and Colo-205 tumor xenografts. [1] Sorafenib treatment produces dose-dependent growth inhibition of PLC/PRF/5 tumor xenografts in SCID mice with TGIs of 49% and 78% at 10 mg/kg and 30 mg/kg, respectively, consistent with the inhibition of ERK and eIF4E phosphorylation, reduction of the microvessel area, and induction of tumor cell apoptosis. [2] Sorafenib sensitizes bax-/- cells to TRAIL in a dose-dependent manner, through a mechanism involving down-regulating NF-κB mediated Mcl-1 and cIAP2 expression. Combining Sorafenib (30-60 mg/kg) with TRAIL (5 mg/kg) show dramatic efficacy in TRAIL-resistant HCT116 bax-/- and HT29 tumor xenografts. [3] |
Protocol (from reference)
| Kinase Assay: |
|
|---|---|
| Cell Assay: |
|
| Animal Study: |
|
References
|
Customer Product Validation
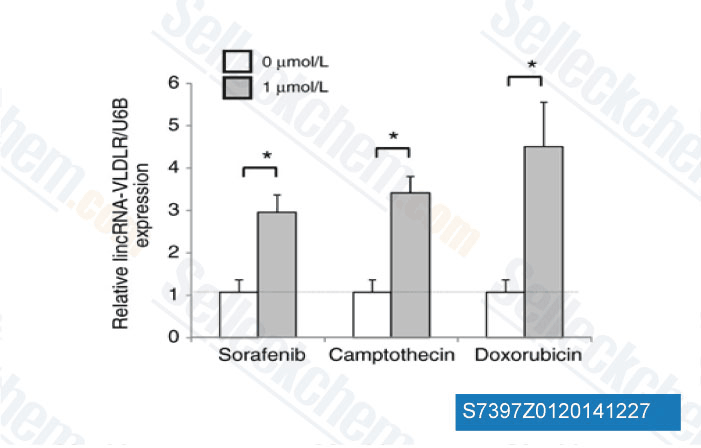
-
Data from [ Mol Cancer Res , 2014 , 12(10), 1377-87 ]
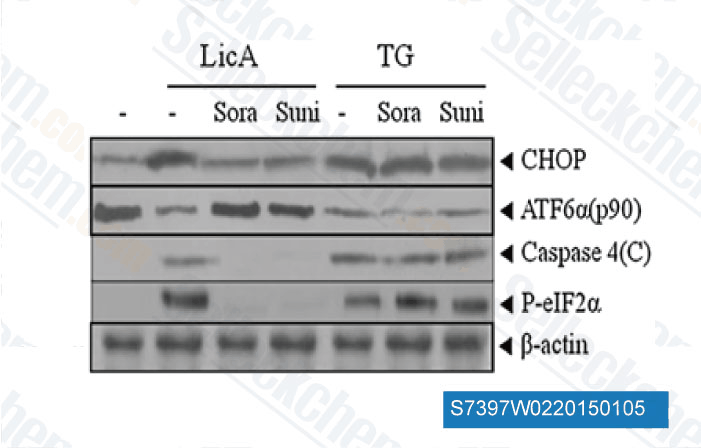
-
Data from [ Apoptosis , 2014 , 19(4), 682-97 ]
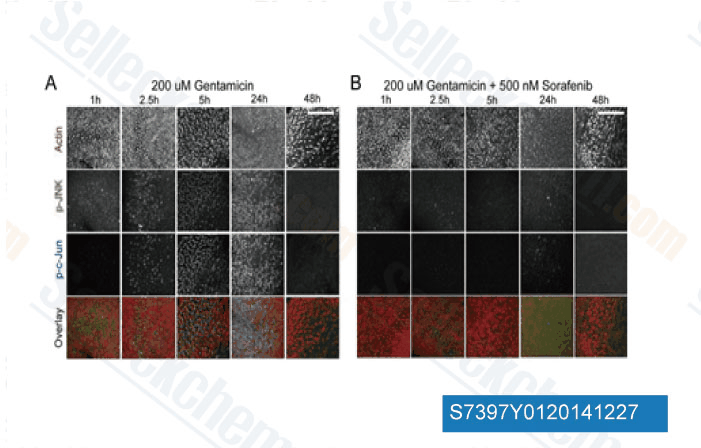
-
Data from [ J Neurosci , 2013 , 33(7), 3079-93 ]
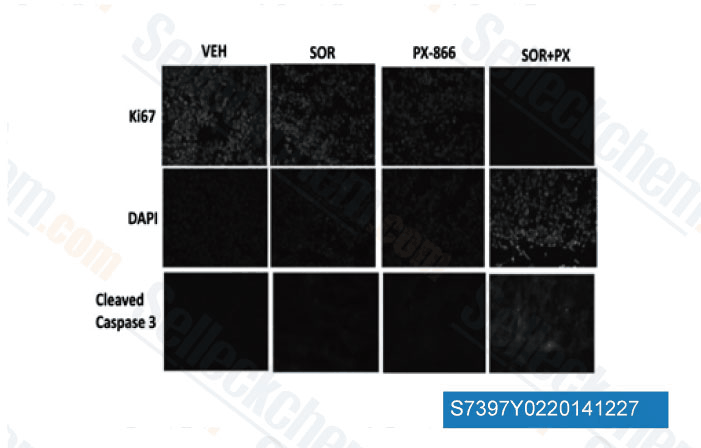
-
Data from [ Mol Pharmacol , 2013 , 84(4), 562-71 ]
Selleck's Sorafenib has been cited by 612 publications
| Sorafenib enhanced the function of myeloid-derived suppressor cells in hepatocellular carcinoma by facilitating PPARα-mediated fatty acid oxidation [ Mol Cancer, 2025, 24(1):34] | PubMed: 39876004 |
| S100P is a ferroptosis suppressor to facilitate hepatocellular carcinoma development by rewiring lipid metabolism [ Nat Commun, 2025, 16(1):509] | PubMed: 39779666 |
| PIP5K1A Suppresses Ferroptosis and Induces Sorafenib Resistance by Stabilizing NRF2 in Hepatocellular Carcinoma [ Adv Sci (Weinh), 2025, 12(30):e04372] | PubMed: 40405713 |
| FLT3 inhibitors induce p53 instability, driven by STAT5/MDM2/p53 competitive interactions in acute myeloid leukemia [ Cancer Lett, 2025, 611:217446] | PubMed: 39756787 |
| In vivo optoacoustic imaging of endothelin receptor expression and treatment response in the hypoxic tumor microenvironment [ Eur J Nucl Med Mol Imaging, 2025, 10.1007/s00259-025-07494-7] | PubMed: 40802092 |
| Matrix stiffness regulates glucose-6-phosphate dehydrogenase expression to mediate sorafenib resistance in hepatocellular carcinoma through the ITGB1-PI3K/AKT pathway [ Cell Death Dis, 2025, 16(1):538] | PubMed: 40685383 |
| Inhibition of Wnt/β-catenin increases anti-tumor activity by synergizing with sorafenib in hepatocellular carcinoma [ Cell Death Dis, 2025, 16(1):466] | PubMed: 40593458 |
| Targeting PTGDS Promotes ferroptosis in peripheral T cell lymphoma through regulating HMOX1-mediated iron metabolism [ Br J Cancer, 2025, 132(4):384-400] | PubMed: 39706989 |
| Targeting the MYC oncogene with a selective bi-steric mTORC1 inhibitor elicits tumor regression in MYC-driven cancers [ Cell Chem Biol, 2025, 32(8):994-1012.e11] | PubMed: 40803322 |
| The Radiosensitizing Effect of Tumor-Derived Microparticles Co-Loaded with Sorafenib and Gold Nanoparticles on Hepatocellular Carcinoma [ Int J Nanomedicine, 2025, 20:5489-5508] | PubMed: 40321799 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
