|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C22H29FN3O9P |
||||||
| Molecular Weight | 529.45 | CAS No. | 1190307-88-0 | ||||
| Solubility (25°C)* | In vitro | DMSO | 100 mg/mL (188.87 mM) | ||||
| Ethanol | 100 mg/mL (188.87 mM) | ||||||
| Water | 13 mg/mL (24.55 mM) | ||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||
Preparing Stock Solutions
Biological Activity
| Description | Sofosbuvir is a HCV NS5B polymerase inhibitor for the treatment of chronic hepatitis C virus (HCV) infection. | |
|---|---|---|
| Targets |
|
|
| In vitro | As HCV NS5B polymerase inhibitor, PSI-7977 displays more potent inhibitory activity against HCV RNA replication than PSI-7976 with EC50 of 92 nM versus 1.07 μM and EC90 of 0.29 μM versus 2.99 μM, consistent with that incubating clone A cells with PSI-7977 leads to a higher concentration of PSI-7409 than clone A cells incubated with PSI-7976. PSI-7977 is an effective substrate for CatA to form PSI-352707 with 18-30 fold more potency as compared with PSI-7976. Unlike GS-7976, however, the CES1-mediated hydrolysis of PSI-7977 does not progress in a time-dependent manner. [1] The S282T NS5B polymerase mutation but not S96T mutation confers resistance to PSI-7977 with EC90 increases from 0.42 μM to 7.8 μM. When assessed in an 8-day cytotoxicity assay, PSI-7977 displays no cytotoxicity against Huh7, HepG2, BxPC3, and CEM cells even at concentrations up to 100 μM. PSI-7977 treatment for 14 days shows a IC90 of 72.1 μM and 68.6 μM for the inhibition of mtDNA and rDNA, respectively, in HepG2 cells. [2] PSI-7977 exhibits potent activity against genotype (GT) 1a, 1b, and 2a (strain JFH-1) replicons and chimeric replicons containing GT 2a (strain J6), 2b, and 3a NS5B polymerase. Sequence analysis of the JFH-1 NS5B region indicates that additional amino acid changes including T179A, M289L, I293L, M434T, and H479P are selected both prior to and after the emergence of S282T, which are required to confer resistance to PSI-7977. [3] |
Protocol (from reference)
| Cell Assay:[2] |
|
|---|
References
|
Customer Product Validation
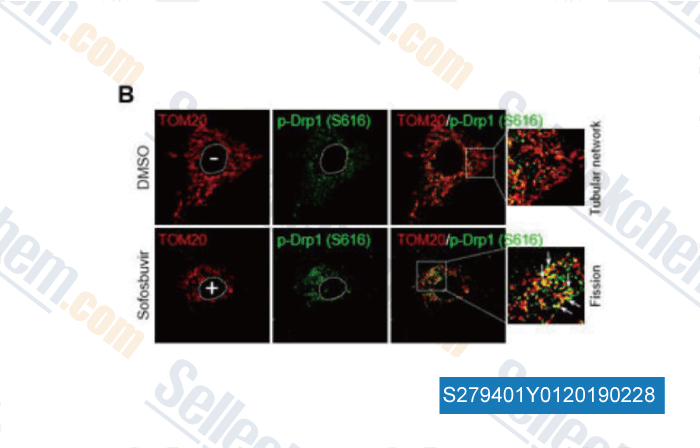
-
Data from [ , , Hepatology, 2017, 66(3):758-771 ]
![Inhibition of hepatitis C virus by sofosbuvir. (A) Determination of 50% cytotoxic concentration (CC50). Huh-7.5 reporter cells (1.3×10<sup>4</sup>) were incubated with the indicated concentration of sofosbuvir (SOF) during 72 h at 37°C, and the numbers of viable cells were counted using MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]. (B) Determination of the drug concentration required for 50% inhibition (IC50) of infectious HCV yield. Huh-7.5 reporter cells (1.0×10<sup>5</sup>) were infected with 3 103 TCID50 of HCV p0 in the presence of the indicated concentrations of SOF. Virus titers were determined in the cell culture supernatants at 72 h postinfection. Viral titers are given as the percentages of the titers obtained in the infection in the absence of SOF. (C) Progeny production in the course of three serial passages of HCV p0 in the presence of increasing concentrations of SOF, as indicated. Infection conditions are those explained in the description of panel B. Procedures are detailed in Materials and Methods.](https://file.selleckchem.com/downloads/review/700px/Sofosbuvir-S279401X0120170116.gif)
-
Data from [ , , Antimicrob Agents Chemother, 2016, 60(6):3786-3793. ]
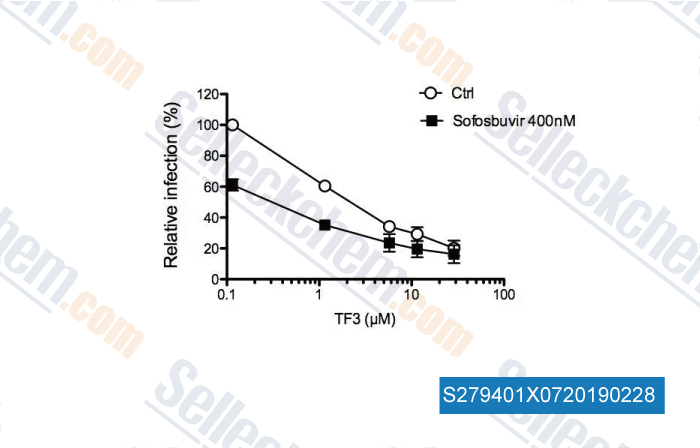
-
Data from [ , , PLoS One, 2018, 13(11):e0198226 ]
Selleck's Sofosbuvir Has Been Cited by 45 Publications
| Rapid hepatitis C virus replication machinery removal after antiviral treatment with DAA monitored by multimodal imaging [ Structure, 2025, S0969-2126(25)00193-5] | PubMed: 40553718 |
| Hepatitis C virus-induced differential transcriptional traits in host cells after persistent infection elimination by direct-acting antivirals in cell culture [ J Med Virol, 2024, 96(7):e29787] | PubMed: 38988177 |
| Immortalized hepatocyte-like cells: A competent hepatocyte model for studying clinical HCV isolate infection [ PLoS One, 2024, 19(5):e0303265] | PubMed: 38739590 |
| Hepatitis C virus infects and perturbs liver stem cells [ mBio, 2023, 10.1128/mbio.01318-23] | PubMed: 37938000 |
| Activation of the urotensin-II receptor by remdesivir induces cardiomyocyte dysfunction [ Commun Biol, 2023, 6(1):511] | PubMed: 37173432 |
| Fitness-Dependent, Mild Mutagenic Activity of Sofosbuvir for Hepatitis C Virus [ Antimicrob Agents Chemother, 2023, 67(7):e0039423] | PubMed: 37367486 |
| ヒト iPS 細胞由来心筋細胞を用いた慢性収縮毒性評価法の開発 [ Okayama University, 2023 , 10.18926/65391] | PubMed: none |
| An anti-influenza A virus microbial metabolite acts by degrading viral endonuclease PA [ Nat Commun, 2022, 13(1):2079] | PubMed: 35440123 |
| Therapeutic targeting of organelles for inhibition of Zika virus replication in neurons [ Antiviral Res, 2022, S0166-3542(22)00233-9] | PubMed: 36396026 |
| Efficacy decrease of antiviral agents when administered to ongoing hepatitis C virus infections in cell culture [ Front Microbiol, 2022, 13:960676] | PubMed: 35992670 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
