|
How to Cite 1. For In-Text Citation (Materials & Methods): 2. For Key Resources Table: |
||
|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. We strive to reply to |
Technical Data
| Formula | C26H27ClN2O3S2 |
||||||||||||||
| Molecular Weight | 515.09 | CAS No. | 115104-28-4 | ||||||||||||
| Solubility (25°C)* | In vitro | DMSO | 100 mg/mL (194.14 mM) | ||||||||||||
| Ethanol | 19 mg/mL (36.88 mM) | ||||||||||||||
| Water | Insoluble | ||||||||||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||||||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||||||||||
Preparing Stock Solutions
Biological Activity
| Description | MK571 is a selective, orally active CysLT1 receptor(Cysteinyl leukotriene receptor) antagonist. | ||||
|---|---|---|---|---|---|
| Targets |
|
||||
| In vitro | L-660,711 inhibited [3H]LTD4 binding in guinea pig lung with a Ki value of 0.22±0.15 nM (n=35) and [3H]LTD4 binding in human lung with a Ki value of 2.1±1.8 nM (n=29). L660,711 was essentially inactive versus [3H]LTC4 binding with IC50 values of 23±11 μM (n=16) and 32 μM (n=1) in guinea pig and human lung, respectively. |
||||
| In vivo | MK-571 is well tolerated in healthy young men. After oral administration, MK-571 is rapidly absorbed. The maximum plasma concentration occurring at 1.1-1.5 h at all doses. |
Protocol (from reference)
| Cell Assay: |
|
|---|---|
| Animal Study: |
|
References
|
Customer Product Validation
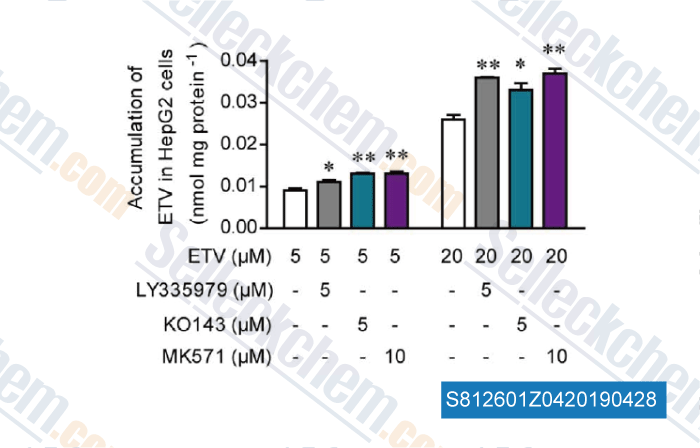
-
Data from [ , , Eur J Pharm Sci, 2017, 106:313-327 ]
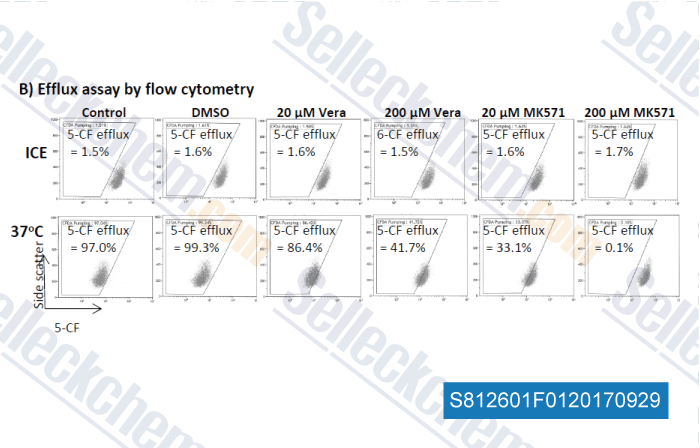
-
Data from [ , , Exp Hematol, 2017, 52:65-71 ]
Selleck's MK-571 (L-660711) Has Been Cited by 14 Publications
| CD142-positive synovial fibroblasts drive meniscus destruction in rheumatoid arthritis [ Nat Commun, 2025, 16(1):6942] | PubMed: 40721606 |
| Protocol to develop a proximal tubule-on-chip model based on hiPSC-derived kidney organoids for functional analysis of renal transporters [ STAR Protoc, 2025, 6(2):103777] | PubMed: 40266846 |
| Establishment and Molecular Characterization of an In Vitro Model for PARPi-Resistant Ovarian Cancer [ Cancers (Basel), 2023, 15(15)3774] | PubMed: 37568590 |
| The Modern Day Heracles: Patient-derived Liver Organoids to Model Rare Pediatric Liver Diseases [ DSpace, 2023, 10.33540/2012] | PubMed: none |
| Transport and Permeation Properties of Dapivirine: Understanding Potential Drug-Drug Interactions [ Pharmaceutics, 2022, 14(9)1948] | PubMed: 36145696 |
| A Histone Deacetylase Inhibitor Induces Acetyl-CoA Depletion Leading to Lethal Metabolic Stress in RAS-Pathway Activated Cells [ Cancers (Basel), 2022, 14(11)2643] | PubMed: 35681624 |
| ABCC5 facilitates the acquired resistance of sorafenib through the inhibition of SLC7A11-induced ferroptosis in hepatocellular carcinoma [ Neoplasia, 2021, 23(12):1227-1239] | PubMed: 34768109 |
| Effect of prednisolone pre-treatment on cat lymphoma cell sensitivity towards chemotherapeutic drugs [ Res Vet Sci, 2021, 138:178-187] | PubMed: 34157499 |
| A thiol-bound drug reservoir enhances APR-246-induced mutant p53 tumor cell death [ EMBO Mol Med, 2020, e10852] | PubMed: 33314700 |
| In vitro Transport Ability of ABCC2 (G1249A) Polymorphic Variant Towards Anticancer Drugs. [ Onco Targets Ther, 2020, 13:1413-1419] | PubMed: 32110040 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
