|
How to Cite 1. For In-Text Citation (Materials & Methods): 2. For Key Resources Table: |
||
|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. We strive to reply to |
Technical Data
| Formula | C10H12FN5O4 |
||||||
| Molecular Weight | 285.23 | CAS No. | 21679-14-1 | ||||
| Solubility (25°C)* | In vitro | DMSO | 57 mg/mL (199.83 mM) | ||||
| Water | Insoluble | ||||||
| Ethanol | Insoluble | ||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||
Preparing Stock Solutions
Biological Activity
| Description | Fludarabine is a STAT1 activation inhibitor which causes a specific depletion of STAT1 protein (and mRNA) but not of other STATs. Also a DNA synthesis inhibitor in vascular smooth muscle cells. Fludarabine induces apoptosis. | |
|---|---|---|
| Targets |
|
|
| In vitro | Fludarabine efficiently inhibits the proliferation of RPMI 8226 cells with IC50 of 1.54 μg/mL. The IC50 of this compound against MM.1S and MM.1R cells is 13.48 μg/mL and 33.79 μg/mL, respectively. In contrast, U266 cells are resistant to this chemical with IC50 of 222.2 μg/mL. This compound treatment results in increased number of cells in the G1 phase of cell cycle, accompanied with a concomitant reduction of cells at the S phase of cell cycle in a time-dependent manner. It induces a cell cycle block and triggers apoptosis in MM cells. It triggers time-dependent cleavage of caspase-8, -9, and -3, -7, followed by PARP cleavage. It increases expression of Bax in a time-dependent fashion, while the expression of Bak doesn't change. After exposure to this chemical for 12 hours, RPMI 8226 cells shows a loss of membrane potential with 61.05% of the cells expressing low fluorescence of rhodamine 123 compared with 8.62% of cells in untreated control. To enhance solubility, it is formulated as the monophosphate (F-ara-AMP, fudarabine), which is instantaneously and quantitatively dephosphorylated to the parent nucleoside upon intravenous infusion. Inside the cells rephosphorylation occurs which leads to fuoroadenine arabinoside triphosphate (F-ara-ATP), the major cytotoxic metabolite of F-ara-A. It can also induce pro-inflammatory stimulation of monocytic cells, as evaluated by increased expression of ICAM-1 and IL-8 release. This compound does not affect the growth of ovarian cancer cell lines, whereas it induces marked and dose-dependent inhibition of proliferation in melanoma cell lines. It is an inhibitor of STAT1 that specifically reduces STAT1 without affecting other STAT family members. In addition to cytoplasmic accumulation, repeated low-dose cisplatin (RLDC) induces HMGB1 expression, which is marked suppressed by STAT1 knockdown. Consistently, this chemical suppresses HMGB1 expression during RLDC treatment dose-dependently in RLDC-treat renal tubular cells. |
|
| In vivo | Tumors treated with PBS grow rapidly to approximately 10-fold their initial volume in 25 day, whereas, the tumors in the Fludarabine at 40 mg/kg increase less than 5-fold. A significant antitumor effect of 40 mg/kg of this compound on RPMI8226 tumor growth is demonstrated. RPMI8226 tumors treated with 40 mg/kg of this chemical at day 10 increase apoptotic nuclei. This compound is effective in suppressing RPMI8226 myeloma xenografts in SCID mice. |
Protocol (from reference)
| Cell Assay: |
|
|---|---|
| Animal Study: |
|
References
|
Customer Product Validation
![Normal human KC pretreated with STAT1 inhibitor (fludarabine [10 uM]) or STAT3 inhibitor (STA-21 [2 uM]) for 24 h. The mRNA levels of hBD2 and hBD3 were assessed by qRT-PCR.](https://file.selleckchem.com/downloads/review/700px/Fludarabine-S1491Z0120141118.gif)
-
Data from [ Mol Cell Biol , 2014 , 34(24), 4368-78. ]
-
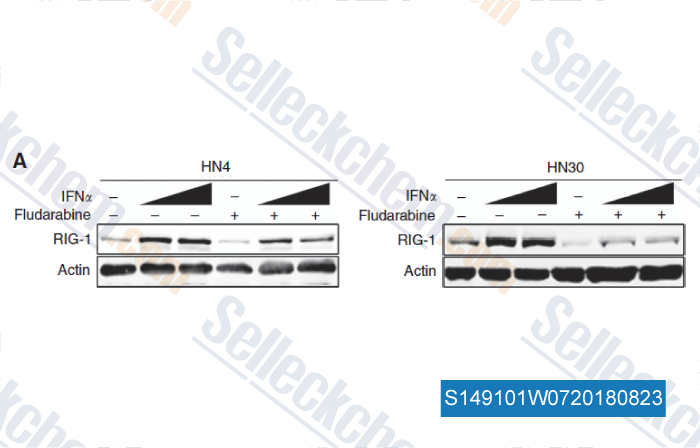
Data from [ , , Br J Cancer, 2018, 118(4):509-521 ]
-
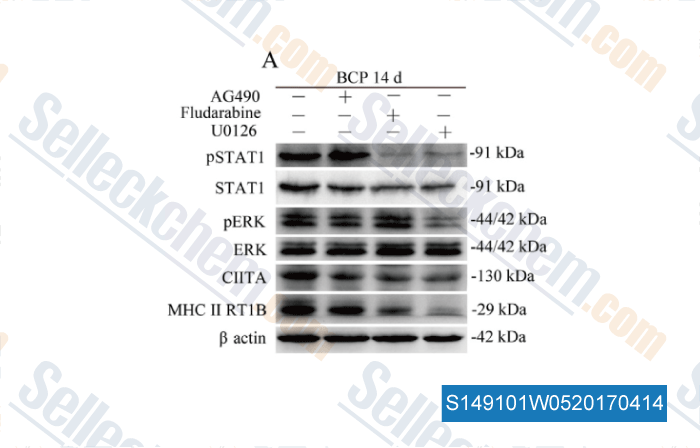
Data from [ , , Brain Behav Immun, 2017, 60:161-173 ]
-
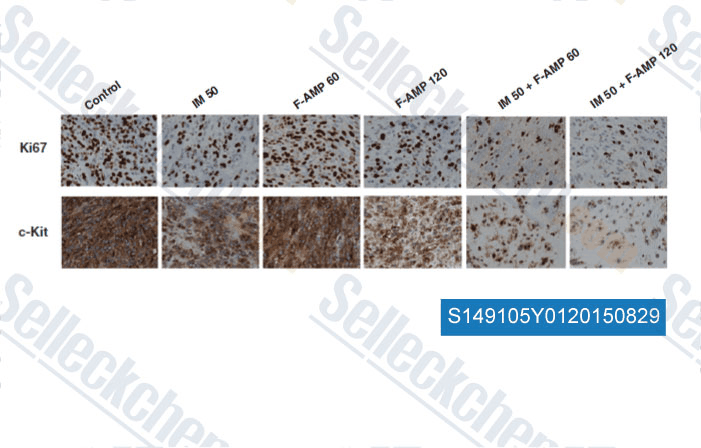
Data from [ , , Mol Cancer Ther, 2014, 13(10): 2276-87 ]
Selleck's Fludarabine Has Been Cited by 209 Publications
| The role of the IL-9‒NLRP3 axis in insulin resistance and adipose tissue inflammation during diet-induced obesity [ Cell Mol Immunol, 2025, 22(11):1478-1490] | PubMed: 40962954 |
| Anti-galectin-9 therapy synergizes with EGFR inhibition to reprogram the tumor microenvironment and overcome immune evasion [ J Immunother Cancer, 2025, 13(7)e010926] | PubMed: 40664443 |
| Cannabinoid CB2 receptor controls chronic itch by regulating spinal microglial activation and synaptic transmission [ Cell Rep, 2025, 44(4):115559] | PubMed: 40222011 |
| STAT1 and STAT6 orchestrate Cbs transcription and transsulfur metabolism in microglia and contribute to parkinson's disease-related neuroinflammation [ Cell Mol Life Sci, 2025, 82(1):294] | PubMed: 40736565 |
| MSC transplantation ameliorates depression in lupus by suppressing Th1 cell-shaped synaptic stripping [ JCI Insight, 2025, e181885] | PubMed: 40048256 |
| Identifying Age-Modulating Compounds Using a Novel Computational Framework for Evaluating Transcriptional Age [ Aging Cell, 2025, e70075] | PubMed: 40307992 |
| Integrated analysis of single-cell RNA-seq and bulk RNA-seq reveal macrophage subpopulation characteristics and the role of STAT1 in rheumatoid arthritis [ J Transl Med, 2025, 23(1):1161] | PubMed: 41131607 |
| Avian leukosis virus subgroup J evades innate immunity by activating miR-155 to dually target TRAF3 and STAT1 [ PLoS Pathog, 2025, 21(10):e1013552] | PubMed: 41066426 |
| Adenosine deaminase and deoxyadenosine regulate intracellular immune response in C. elegans [ iScience, 2025, 28(3):111950] | PubMed: 40034845 |
| Polyinosinic-polycytidylic acid modulates Porphyromonas gingivalis-induced cell apoptosis via the janus kinase/ signal transducer and activator of transcription signaling pathway [ J Dent Sci, 2025, 20(2):811-818] | PubMed: 40224116 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
