|
How to Cite 1. For In-Text Citation (Materials & Methods): 2. For Key Resources Table: |
||
|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. We strive to reply to |
Technical Data
| Formula | C22H18Cl2N8 |
||||||
| Molecular Weight | 465.34 | CAS No. | 252917-06-9 | ||||
| Solubility (25°C)* | In vitro | DMSO | 93 mg/mL (199.85 mM) | ||||
| Water | Insoluble | ||||||
| Ethanol | Insoluble | ||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||
Preparing Stock Solutions
Biological Activity
| Description | Laduviglusib (CHIR-99021, CT99021) is a GSK-3α and GSK-3β inhibitor with IC50 values of 10 nM and 6.7 nM, respectively. It does not exhibit cross-reactivity against cyclin-dependent kinases (CDKs) and shows a 350-fold selectivity toward GSK-3β compared to CDKs. This compound functions as a Wnt/β-catenin activator and induces autophagy. | ||||
|---|---|---|---|---|---|
| Targets |
|
||||
| In vitro | CHIR-99021 (Laduviglusib) shows greater than 500-fold selectivity for GSK-3 versus its closest homologs CDC2 and ERK2, as well as other protein kinases. Furthermore, it shows only weak binding to a panel of 22 pharmacologically relevant receptors and little inhibitory activity against a panel of 23 nonkinase enzymes. This compound induces the activation of glycogen synthase (GS) in insulin receptor-expressing CHO-IR cells with EC50 of 0.763 μM[1]. | ||||
| In vivo | Oral administration of CHIR-99021 (Laduviglusib) at 30 mg/kg enhances glucose metabolism in a rodent model of type 2 diabetes, with a maximal plasma glucose reduction of nearly 150 mg/dl 3-4 hours after administration, while plasma insulin remains at or below control levels. When given orally at 16 or 48 mg/kg 1 hour before oral glucose challenges in ZDF rats, this compound significantly improves glucose tolerance with 14% and 33% reduction in plasma glucose at 16 mg/kg and 48 mg/kg, respectively; the higher dose also reduces hyperglycemia before the oral glucose challenge[1]. |
Protocol (from reference)
| Cell Assay: |
|
|---|---|
| Animal Study: |
|
References
|
Customer Product Validation
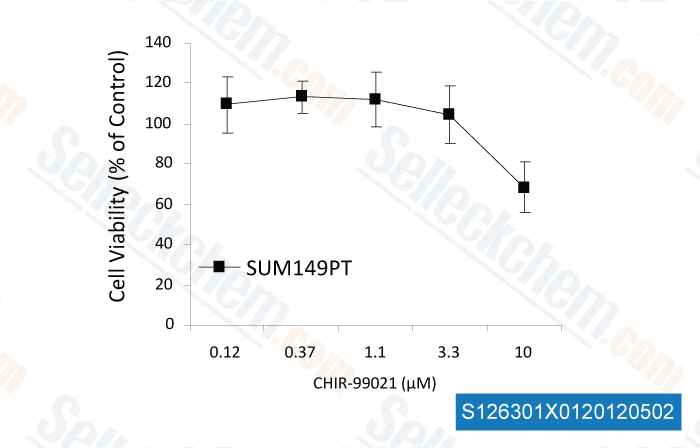
-
, , Dr. Yong-Weon Yi from Georgetown University Medical Center
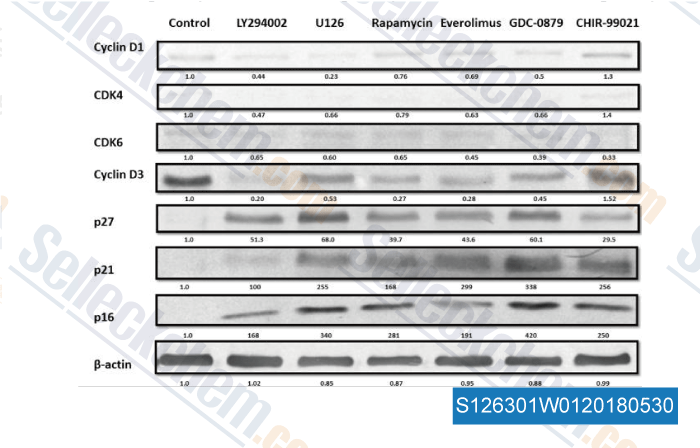
-
Data from [ , , Med Oncol, 2017, 35(1):7 ]
Selleck's CHIR-99021 (Laduviglusib) Has Been Cited by 1134 Publications
| Proteogenomic characterization of non-functional pancreatic neuroendocrine tumors unravels clinically relevant subgroups [ Cancer Cell, 2025, 43(4):776-796.e14] | PubMed: 40185092 |
| Olmesartan Restores LMNA Function in Haploinsufficient Cardiomyocytes [ Circulation, 2025, 151(20):1436-1448] | PubMed: 40166828 |
| Olmesartan Restores LMNA Function in Haploinsufficient Cardiomyocytes [ Circulation, 2025, 151(20):1436-1448] | PubMed: 40166828 |
| Structure-guided discovery of highly efficient cytidine deaminases with sequence-context independence [ Nat Biomed Eng, 2025, 9(1):93-108] | PubMed: 38831042 |
| The nuclear periphery confers repression on H3K9me2-marked genes and transposons to shape cell fate [ Nat Cell Biol, 2025, 27(8):1311-1326] | PubMed: 40696106 |
| A continuous totipotent-like cell-based embryo model recapitulates mouse embryogenesis from zygotic genome activation to gastrulation [ Nat Cell Biol, 2025, NONE] | PubMed: 41094030 |
| Targeting Cardiomyocyte PCNA and POLD1 Prevents Pathologic Myocardial Hypertrophy [ Circ Res, 2025, 137(9):1160-1181] | PubMed: 40948130 |
| Microprotein PLUM encoded by Lin28b uORF is a cytoplasmic determinant of pluripotency and embryonic development [ Nat Commun, 2025, 16(1):10324] | PubMed: 41298451 |
| Identification of functional non-coding variants associated with orofacial cleft [ Nat Commun, 2025, 16(1):6545] | PubMed: 40670354 |
| AAV9-mediated MYBPC3 gene therapy with optimized expression cassette enhances cardiac function and survival in MYBPC3 cardiomyopathy models [ Nat Commun, 2025, 16(1):2196] | PubMed: 40038304 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
