|
How to Cite 1. For In-Text Citation (Materials & Methods): 2. For Key Resources Table: |
||
|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. We strive to reply to |
Technical Data
| Formula | C23H28N8OS |
||||||
| Molecular Weight | 464.59 | CAS No. | 639089-54-6 | ||||
| Solubility (25°C)* | In vitro | DMSO | 93 mg/mL (200.17 mM) | ||||
| Water | Insoluble | ||||||
| Ethanol | Insoluble | ||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||
Preparing Stock Solutions
Biological Activity
| Description | Tozasertib (VX-680) is a pan-Aurora inhibitor, mostly against Aurora A with Kiapp of 0.6 nM in a cell-free assay, less potent towards Aurora B/Aurora C and 100-fold more selective for Aurora A than 55 other kinases. The only exceptions are Fms-related tyrosine kinase-3 (FLT-3) and BCR-ABL tyrosine kinase, which are inhibited by this compound with both Ki of 30 nM. It induces apoptosis and autophagy, and is in Phase 2. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Targets |
|
||||||||||
| In vitro | Although its multi-kinase profile, Tozasertib (VX-680) induces similar cytotoxicity with IC50 of approximately 300 nM and exhibits an AUR B-like inhibitory phenotype of G2/M arrest, endoreduplication and apoptosis in BaF3 cells transfected with ABL or FLT-3 (mutant and wild type) kinases. This compound prevents the CAL-62 proliferation in a time-dependent manner. Treatment for 14 days significantly decreases the number and size of colonies by approximately 70% in the 8305C and 90% in the CAL-62, 8505C and BHT-101. Treatment of the different ATC cells with it inhibits proliferation with the IC50 between 25 and 150 nM. It significantly impairs the ability of the different cell lines to form colonies in soft agar. Analysis of caspase-3 activity indicates that VX-680 induces apoptosis in the different cell lines. CAL-62 cells exposed for 12 hours to the compound showed an accumulation of cells with ≥4N DNA content. Time-lapse analysis demonstrates that treated CAL-62 cells exit metaphase without dividing. Moreover, histone H3 phosphorylation is abrogated following its treatment. It has significant inhibitory activity against BCR-Abl bearing the T315I mutation in patient-derived samples. | ||||||||||
| In vivo | Tozasertib (VX-680) gives rise to a marked decrease in tumor size in a human AML (HL-60) xenograft model. In mude mice treateed with it at 75 mg/kg, twice a day intraperitoneally (b.i.d. i.p.) for 13 days, mean tumor volumes are reduced by 98%. Tumor growth decrease is dose dependent and significant at a dose of 12.5 mg/kg b.i.d. This compound is well tolerated, with a small decrease in body weight observed only at the highest dose. It also triggers tumor regresson in pancreatic and colon xenograft models. Tozasertib also displays potent antitumor activity when infused i.v. in mude rats bearing established HCT116 tumors. A higher dose (2 mg/kg/h) improves efficacy with a 56% decrease in mean tumor volume. |
Protocol (from reference)
| Kinase Assay: |
|
|---|---|
| Cell Assay: |
|
| Animal Study: |
|
References
|
Customer Product Validation
-
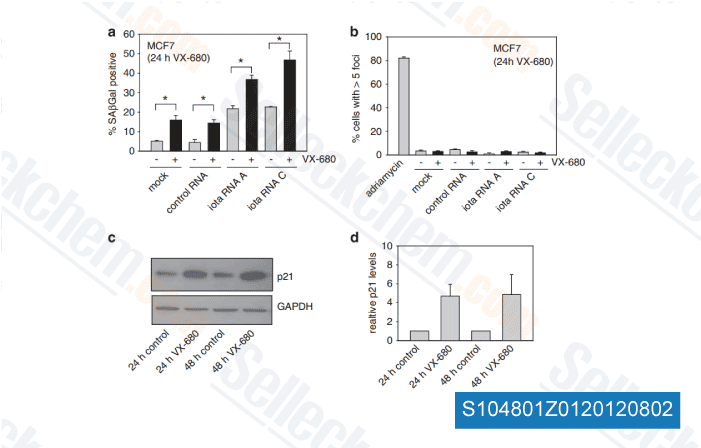
Data from [ Oncogene , 2012 , 31, 3584-96 ]

-
Data from [ Oncogene , 2012 , 31, 3584-96 ]
-
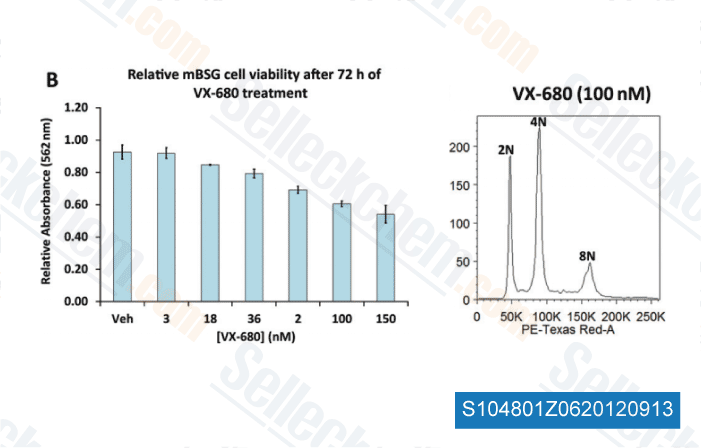
Data from [ Brain Pathol , 2012 , 23, 244-53 ]
-
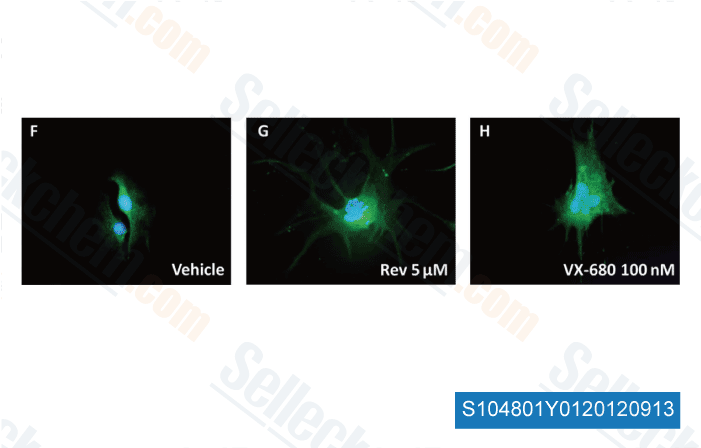
Data from [ Brain Pathol , 2012 , 23, 244-53 ]
Selleck's Tozasertib (VX-680) Has Been Cited by 139 Publications
| A patient-derived T cell lymphoma biorepository uncovers pathogenetic mechanisms and host-related therapeutic vulnerabilities [ Cell Rep Med, 2025, S2666-3791(25)00102-8] | PubMed: 40147445 |
| Overcoming MET-targeted drug resistance in MET-amplified lung cancer by aurora kinase B inhibition [ Biochim Biophys Acta Mol Cell Res, 2025, 1872(7):120001] | PubMed: 40499687 |
| Hedgehog signalling is involved in acquired resistance to KRASG12C inhibitors in lung cancer cells [ Cell Death Dis, 2024, 15(1):56] | PubMed: 38225225 |
| Dynamic phosphorylation of FOXA1 by Aurora B guides post-mitotic gene reactivation [ Cell Rep, 2024, 43(9):114739] | PubMed: 39276350 |
| The molecular basis of Abelson kinase regulation by its αI-helix [ Elife, 2024, 12RP92324] | PubMed: 38588001 |
| Tozasertib activates anti-tumor immunity through decreasing regulatory T cells in melanoma [ Neoplasia, 2024, 48:100966] | PubMed: 38237304 |
| Machine learning based androgen receptor regulatory gene-related random forest survival model for precise treatment decision in prostate cancer [ Heliyon, 2024, 10(17):e37256] | PubMed: 39296076 |
| Visualization strategies to aid interpretation of high-dimensional genotoxicity data [ Environ Mol Mutagen, 2024, 10.1002/em.22604] | PubMed: 38757760 |
| Molecular landscape and functional characterization of centrosome amplification in ovarian cancer [ Nat Commun, 2023, 14(1):6505] | PubMed: 37845213 |
| Mitotic Dysregulation at Tumor Initiation Creates a Therapeutic Vulnerability to Combination Anti-Mitotic and Pro-Apoptotic Agents for MYCN-Driven Neuroblastoma [ Int J Mol Sci, 2023, 10.3390/ijms242115571] | PubMed: 37958555 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
