|
How to Cite 1. For In-Text Citation (Materials & Methods): 2. For Key Resources Table: |
||
|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. We strive to reply to |
Technical Data
| Formula | C31H42N6O4 |
||||||
| Molecular Weight | 562.7 | CAS No. | 387867-13-2 | ||||
| Solubility (25°C)* | In vitro | DMSO | 100 mg/mL (177.71 mM) | ||||
| Ethanol | 100 mg/mL (177.71 mM) | ||||||
| Water | Insoluble | ||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||
Preparing Stock Solutions
Biological Activity
| Description | Tandutinib (MLN518, CT53518, NSC726292) is a potent FLT3 antagonist with IC50 of 0.22 μM. This compound also inhibits PDGFR and c-Kit, with 15 to 20-fold higher potency for FLT3 versus CSF-1R and >100-fold selectivity for the same target versus FGFR, EGFR and KDR. It was studied in Phase 2. | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Targets |
|
||||||||
| In vitro | Tandutinib (MLN518) has little activity against EGFR, FGFR, KDR, InsR, Src, Abl, PKC, PKA and MAPKs. It inhibits IL-3-independent cell growth and FLT3-ITD autophosphorylation with an IC50 of 10-100 nM. This compound also inhibits the proliferation of human leukemia Ba/F3 cells containing FLT3-ITD mutations with IC50 values of 10-30 nM, and the FLT3-ITD-positive Molm-13 and Molm-14 cells with an IC50 of 10 nM. In FLT3-ITD-positive Molm-14 cells but not the FLT3-ITD-negative THP-1 cells, Tandutinib treatment leads to significant apoptosis by 51% and 78% at 24 and 96 hours, respectively, due to specific FLT3 inhibition. It preferentially inhibits the growth of blast colonies from FLT3 ITD-positive compared with ITD-negative patients with AML, without affecting colony formation by normal human progenitor cells. |
||||||||
| In vivo | Oral administration of Tandutinib (MLN518) at 60 mg/kg bid significantly increases the survival in mice bearing Ba/F3 cells expressing W51 FLT3-ITD mutant, and gives a significant reduction in mortality in a mouse bone marrow transplantation model. Treatment with this compound at 180 mg/kg twice daily has mild toxicity toward normal hematopoiesis, however, it is a dose at which it is effective in treating FLT3 ITD-positive leukemia in mice. |
Protocol (from reference)
| Kinase Assay: |
|
|---|---|
| Cell Assay: |
|
| Animal Study: |
|
References
|
Customer Product Validation
-
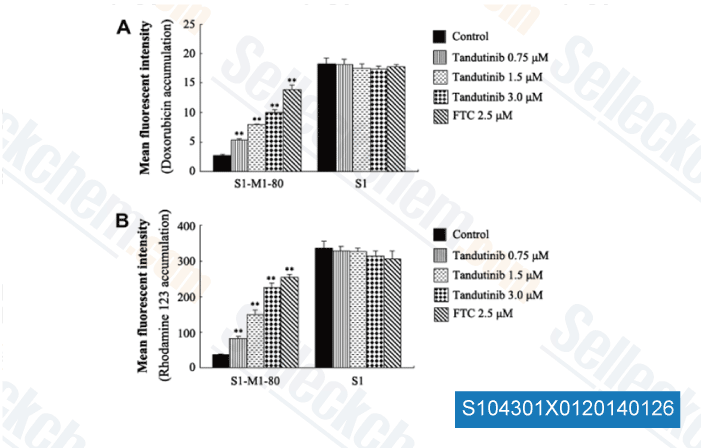
Data from [ Eur J Pharm Sci , 2013 , 49(3), 441-50 ]
-
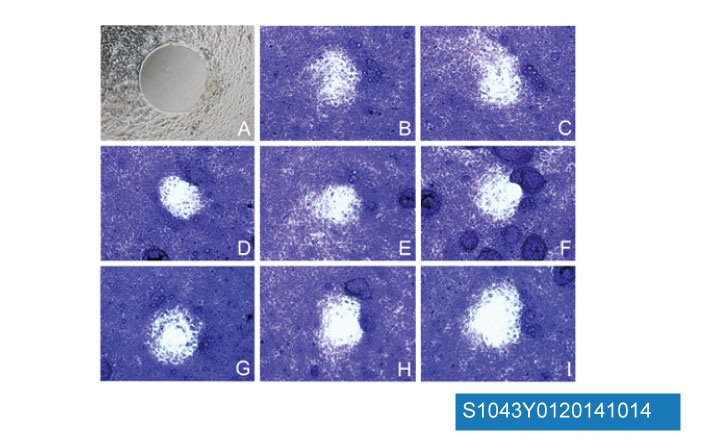
Data from [ Br J Cancer , 2012 , 107, 1702-13 ]
Selleck's Tandutinib (MLN518) Has Been Cited by 14 Publications
| Proteogenomic characterization identifies clinically relevant subgroups of intrahepatic cholangiocarcinoma [ Cancer Cell, 2021, S1535-6108(21)00659-0] | PubMed: 34971568 |
| Extension of the Mechanistic Tissue Distribution Model of Rodgers and Rowland by Systematic Incorporation of Lysosomal Trapping: Impact on Unbound Partition Coefficient and Volume of Distribution Predictions in the Rat [ Drug Metab Dispos, 2021, 49(1):53-61] | PubMed: 33148688 |
| Comprehensive pharmacogenomic characterization of gastric cancer. [ Genome Med, 2020, 18;12(1):17] | PubMed: 32070411 |
| Small-Molecule and CRISPR Screening Converge to Reveal Receptor Tyrosine Kinase Dependencies in Pediatric Rhabdoid Tumors. [ Cell Rep, 2019, 28(9):2331-2344] | PubMed: 31461650 |
| Small Molecule Amyloid-β Protein Precursor Processing Modulators Lower Amyloid-β Peptide Levels via cKit Signaling. [ J Alzheimers Dis, 2019, 67(3):1089-1106] | PubMed: 30776010 |
| MAST1 Drives Cisplatin Resistance in Human Cancers by Rewiring cRaf-Independent MEK Activation [ Cancer Cell, 2018, 34(2):315-330] | PubMed: 30033091 |
| Palbociclib treatment of FLT3-ITD+ AML cells uncovers a kinase-dependent transcriptional regulation of FLT3 and PIM1 by CDK6 [Uras IZ, et al. Blood, 2016, 127(23):2890-902] | PubMed: 27099147 |
| Metabolic alterations and drug sensitivity of tyrosine kinase inhibitor resistant leukemia cells with a FLT3/ITD mutation [Huang A, et al. Cancer Lett, 2016, 377(2):149-57] | PubMed: 27132990 |
| Discovery and Rational Design of Pteridin-7(8H)-one-Based Inhibitors Targeting FMS-like Tyrosine Kinase 3 (FLT3) and Its Mutants. [ J Med Chem, 2016, 59(13):6187-200] | PubMed: 27266526 |
| Targeting a cell state common to triple-negative breast cancers [Muellner MK, et al. Mol Syst Biol, 2015, 11(1):789] | PubMed: 25699542 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
