|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C22H27FN4O2.C4H6O5 |
||||||||||
| Molecular Weight | 532.56 | CAS No. | 341031-54-7 | ||||||||
| Solubility (25°C)* | In vitro | DMSO | 70 mg/mL (131.44 mM) | ||||||||
| Water | Insoluble | ||||||||||
| Ethanol | Insoluble | ||||||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||||||
Preparing Stock Solutions
Biological Activity
| Description | Sunitinib malate is a multi-targeted RTK inhibitor targeting VEGFR2 (Flk-1) and PDGFRβ with IC50 of 80 nM and 2 nM in cell-free assays, and also inhibits c-Kit. Sunitinib Malate effectively inhibits autophosphorylation of Ire1α. Sunitinib Malate increases both death receptor and mitochondrial-dependent apoptosis. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Targets |
|
||||||||||
| In vitro | Sunitinib also potently inhibits Kit and FLT-3. [1] Sunitinib is a potent ATP-competitive inhibitor of VEGFR2 (Flk1) and PDGFRβ with Ki of 9 nM and 8 nM, respectively, displaying >10-fold higher selectivity for VEGFR2 and PDGFR than FGFR-1, EGFR, Cdk2, Met, IGFR-1, Abl, and src. In serum-starved NIH-3T3 cells expressing VEGFR2 or PDGFRβ, Sunitinib inhibits VEGF-dependent VEGFR2 phosphorylation and PDGF-dependent PDGFRβ phosphorylation with IC50 of 10 nM and 10 nM, respectively. Sunitinib inhibits VEGF-induced proliferation of serum-starved HUVECs with IC50 of 40 nM, and inhibits PDGF-induced proliferation of NIH-3T3 cells overexpressing PDGFRβ or PDGFRα with IC50 of 39 nM and 69 nM, respectively. [2] Sunitinib inhibits phosphorylation of wild-type FLT3, FLT3-ITD, and FLT3-Asp835 with IC50 of 250 nM, 50 nM, and 30 nM, respectively. Sunitinib inhibits the proliferation of MV4;11 and OC1-AML5 cells with IC50 of 8 nM and 14 nM, respectively, and induces apoptosis in a dose-dependent manner. [3] |
||||||||||
| In vivo | Consistent with the substantial and selective inhibition of VEGFR2 or PDGFR phosphorylation and signaling in vivo, Sunitinib (20-80 mg/kg/day) exhibits broad and potent dose-dependent anti-tumor activity against a variety of tumor xenograft models including HT-29, A431, Colo205, H-460, SF763T, C6, A375, or MDA-MB-435. Sunitinib dosing at 80 mg/kg/day for 21 days leads to complete tumor regression in six of eight mice, without tumor re-growing during a 110-day observation period after the end of treatment. Second round of treatment with Sunitinib remains efficacious against tumors that are not fully regressed during the first round of treatment. Sunitinib treatment results in significant decrease in tumor MVD, with ~40% reduction in SF763T glioma tumors. SU11248 treatment results in a complete inhibition of additional tumor growth of luciferase-expressing PC-3M xenografts, despite no reduction in tumor size. [2] Sunitinib treatment (20 mg/kg/day) dramatically suppresses the growth subcutaneous MV4;11 (FLT3-ITD) xenografts and prolongs survival in the FLT3-ITD bone marrow engraftment model. [3] |
Protocol (from reference)
| Kinase Assay: |
|
|---|---|
| Cell Assay: |
|
| Animal Study: |
|
References
|
Customer Product Validation
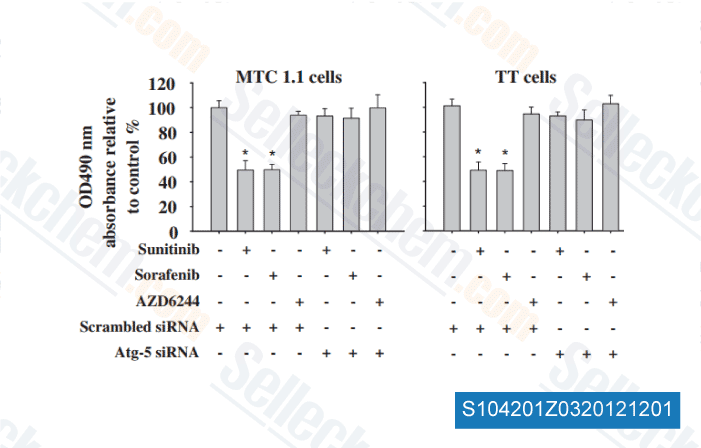
-
Data from [ Surgery , 2012 , 152, 1142-9 ]
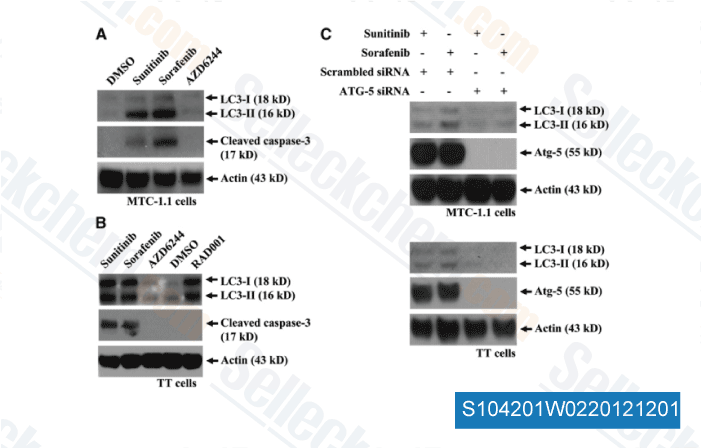
-
Data from [ Surgery , 2012 , 152, 1142-9 ]
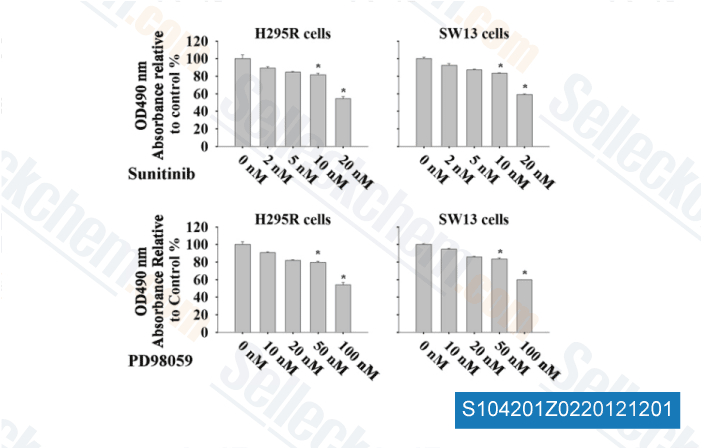
-
Data from [ Surgery , 2012 , 152, 1045-50 ]
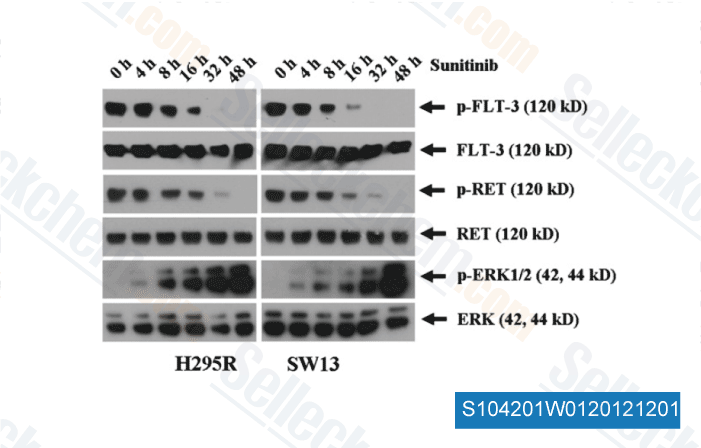
-
Data from [ Surgery , 2012 , 152, 1045-50 ]
Selleck's Sunitinib malate Has Been Cited by 177 Publications
| Biofabrication of pheochromocytoma and paraganglioma tumor organoids and assessment of response to systemic therapy [ Sci Rep, 2025, 15(1):35889] | PubMed: 41087622 |
| Establishment, characterization, and biobanking of 36 pancreatic cancer organoids: prediction of metastasis in resectable pancreatic cancer [ Cell Oncol (Dordr), 2024, 10.1007/s13402-024-00939-5] | PubMed: 38619751 |
| Patient-derived rhabdomyosarcoma cells recapitulate the genetic and transcriptomic landscapes of primary tumors [ iScience, 2024, 27(10):110862] | PubMed: 39319271 |
| Canthin-6-One Inhibits Developmental and Tumour-Associated Angiogenesis in Zebrafish [ Pharmaceuticals (Basel), 2024, 17(1)108] | PubMed: 38256941 |
| Impact of sunitinib resistance on clear cell renal cell carcinoma therapeutic sensitivity in vitro [ Cell Cycle, 2024, 1-13.] | PubMed: 38263737 |
| Multi-omics and immunogenomics analysis revealed PFKFB3 as a targetable hallmark and mediates sunitinib resistance in papillary renal cell carcinoma: in silico study with laboratory verification [ Eur J Med Res, 2024, 29(1):236] | PubMed: 38622715 |
| Elucidation and Regulation of Tyrosine Kinase Inhibitor Resistance in Renal Cell Carcinoma Cells from the Perspective of Glutamine Metabolism [ Metabolites, 2024, 14(3)170] | PubMed: 38535330 |
| Disruption of Autophagic Flux and Treatment with the PDPK1 Inhibitor GSK2334470 Synergistically Inhibit Renal Cell Carcinoma Pathogenesis [ J Cancer, 2024, 15(5):1429-1441] | PubMed: 38356720 |
| Extracellular Vesicle-Mediated Transfer of LncRNA IGFL2-AS1 Confers Sunitinib Resistance in Renal Cell Carcinoma [ Cancer Res, 2023, 83(1):103-116] | PubMed: 36264173 |
| Anti-angiogenic therapy using the multi-tyrosine kinase inhibitor Regorafenib enhances tumor progression in a transgenic mouse model of ß-cell carcinogenesis [ Br J Cancer, 2023, 129(8):1225-1237] | PubMed: 37620408 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
