|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C25H38O5 |
||||||
| Molecular Weight | 418.57 | CAS No. | 79902-63-9 | ||||
| Solubility (25°C)* | In vitro | DMSO | 84 mg/mL (200.68 mM) | ||||
| Ethanol | 84 mg/mL (200.68 mM) | ||||||
| Water | Insoluble | ||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||
Preparing Stock Solutions
Biological Activity
| Description | Simvastatin is a competitive inhibitor of HMG-CoA reductase with Ki of 0.1-0.2 nM in cell-free assays. Simvastatin induces ferroptosis, mitophagy, autophagy and apoptosis. | ||
|---|---|---|---|
| Targets |
|
||
| In vitro | Prior to use in cell assays, Simvastatin needs to be activated by NaOH in EtOH treatment. Simvastatin inhibits cholesterol synthesis in mouse L-M cell (fibroblast), rat H4II E cell (liver), and human Hep G2 cell (liver) with IC50 of 19.3 nM, 13.3 nM and 15.6 nM, respectively. [1] Simvastatin treatment leads to a dose-dependent increase in serine 473 phosphorylation of Akt within 30 minutes, with maximal phosphorylation occurring at 1.0 µM. Simvastatin (1.0 μM) enhances phosphorylation of the endogenous Akt substrate endothelial nitric oxide synthase (eNOS), inhibits serum-free media undergo apoptosis and accelerates vascular structure formation. [2] Simvastatin displays anti-inflammatory effects in vitro. Simvastatin (10 μM) reduces anti-CD3/anti-CD28 antibody-stimulated proliferation of PB-derived mononuclear cells and synovial fluid cells from rheumatoid arthritis blood, as well as IFN-γ release. Simvastatin (10 μM) suppresses cell-mediated macrophage TNF-γ release induced via cognate interactions by ~30%. [3] |
||
| In vivo | Simvastatin orally administration inhibits the conversion of radiolabeled acetate to cholesterol with IC50 of 0.2 mg/kg. [1] Simvastatin (4 mg/day) orally administration for 13 weeks to rabbits fed an atherogenci cholesterol-rich diet, returns the cholesterol-induced increases in total cholesterol, LDL-cholesterol and HDL-cholesterol to normal level. [4] Simvastatin (6 mg/kg) produces an increase in LDL receptor-dependent binding and increases the number of hepatic LDL receptors in rabbits fed a diet containing 0.25% cholesterol. [5] Simvastatin influences inflammation independent of its effect on plasma cholesterol level. In cynomolgus monkeys consumed an atherogenic diet, Simvastatin (20 mg/kg/day) induces a 1.3-fold less macrophage content in lesions, and 2-fold less vascular cell adhesion molecule-1, interleukin-1beta, and tissue factor expression, companied by a 2.1-fold increases in lesional smooth muscle cell and collagen content. [6] |
Protocol (from reference)
| Kinase Assay: |
|
|---|---|
| Cell Assay: |
|
| Animal Study: |
|
References
|
Customer Product Validation
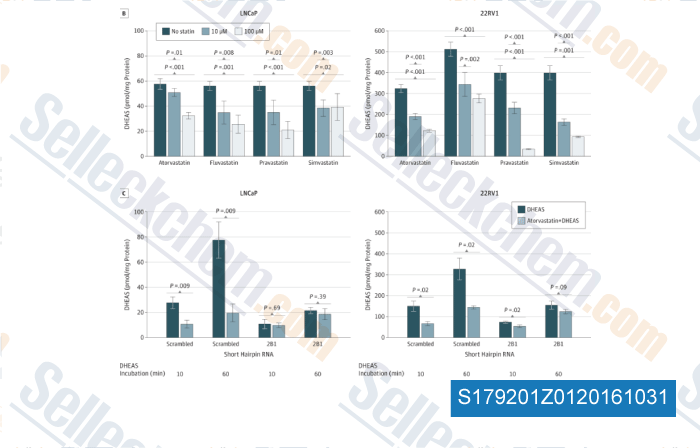
-
, , JAMA Oncol, 2015, 1(4):495-504.
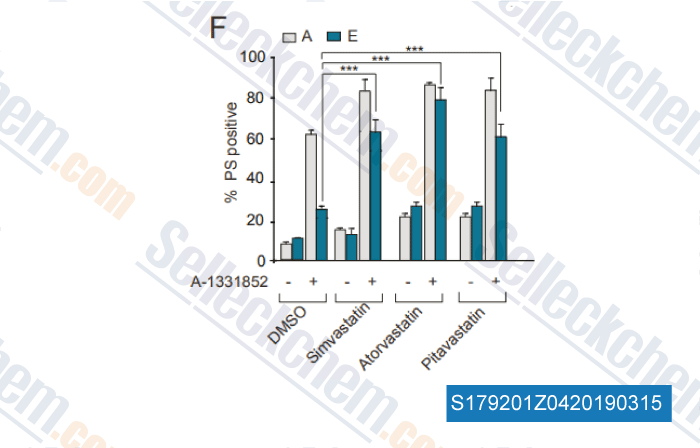
-
Data from [ , , Haematologica, 2018, doi: 10.3324/haematol.2018.204701 ]
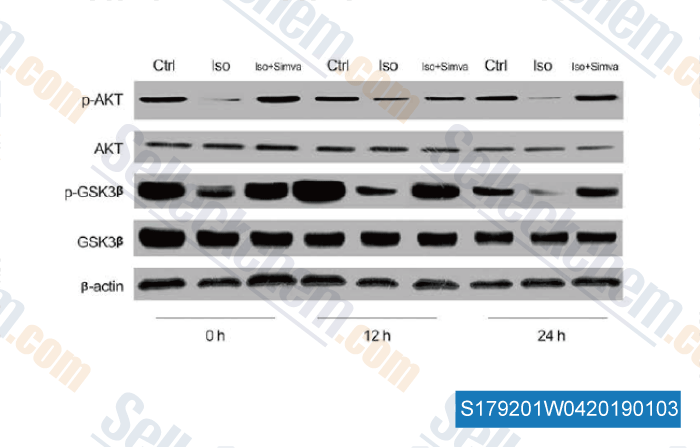
-
Data from [ , , Cell Physiol Biochem, 2018, 46(2):618-632 ]
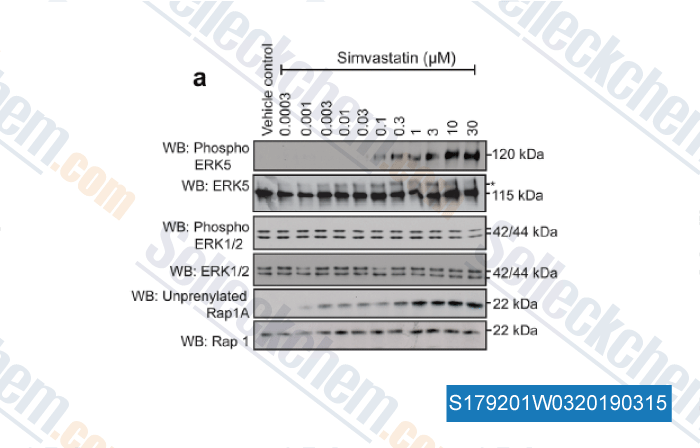
-
Data from [ , , J Cell Physiol, 2018, 233(1):186-200 ]
Selleck's Simvastatin Has Been Cited by 82 Publications
| Simvastatin overcomes the pPCK1-pLDHA-SPRINGlac axis-mediated ferroptosis and chemo-immunotherapy resistance in AKT-hyperactivated intrahepatic cholangiocarcinoma [ Cancer Commun (Lond), 2025, 10.1002/cac2.70036] | PubMed: 40443016 |
| Coenzyme A protects against ferroptosis via CoAlation of mitochondrial thioredoxin reductase [ J Clin Invest, 2025, e190215] | PubMed: 40694424 |
| Commonly prescribed multi-medication therapies exert sex-specific effects on Alzheimer's disease pathology and metabolomic profiles in AppNL-G-F mice: Implications for personalized therapeutics in aging [ Alzheimers Dement, 2025, 21(3):e70081] | PubMed: 40145346 |
| Macrophages form dendrite-like pseudopods to enhance bacterial ingestion [ EMBO J, 2025, 10.1038/s44318-025-00515-z] | PubMed: 40721684 |
| CRISPR/Cas9-mediated SHP-1-knockout T cells combined with simvastatin enhances anti-tumor activity in humanized-PDX HCC model [ iScience, 2025, 28(4):112266] | PubMed: 40241752 |
| Ferroptosis as a therapeutic vulnerability in MDM2 inhibition in dedifferentiated liposarcoma [ Oncol Lett, 2025, 29(6):269] | PubMed: 40247991 |
| Epstein-Barr Virus-Driven B-Cell Transformation under Germinal Center Hypoxia Requires External Unsaturated Fatty Acids [ Res Sq, 2025, rs.3.rs-6506954] | PubMed: 40313738 |
| The mevalonate pathway couples lipid metabolism to amino acid synthesis via ubiquinone-dependent redox control [ bioRxiv, 2025, 2025.08.10.669191] | PubMed: 40832310 |
| ETS1 Orchestrates a Hybrid EMT Program Driving in vivo Metastasis and Immune Evasion [ bioRxiv, 2025, 2025.07.17.665404] | PubMed: 40777467 |
| Propafenone facilitates mitochondrial-associated ferroptosis and synergizes with immunotherapy in melanoma [ J Immunother Cancer, 2024, 12(11)e009805] | PubMed: 39581704 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
