|
How to Cite 1. For In-Text Citation (Materials & Methods): 2. For Key Resources Table: |
||
|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. We strive to reply to |
Technical Data
| Formula | C32H31F2N3O2.3HCl |
||||||||||
| Molecular Weight | 636.99 | CAS No. | 167465-36-3 | ||||||||
| Solubility (25°C)* | In vitro | DMSO | 100 mg/mL (156.98 mM) | ||||||||
| Water | 28 mg/mL (43.95 mM) | ||||||||||
| Ethanol | Insoluble | ||||||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||||||
Preparing Stock Solutions
Biological Activity
| Description | Zosuquidar 3HCl is a potent modulator of P-glycoprotein-mediated multi-drug resistance with Ki of 60 nM in a cell-free assay. Phase 3. | ||
|---|---|---|---|
| Targets |
|
||
| In vitro | LY335979 competitively inhibits equilibrium binding of Pgp by blocking [3H]azidopine photoaffinity labeling of the Pgp in CEM/VLB100 plasma membranes. LY335979 alone shows the cytotoxicity to drug-sensitive and MDR cell lines with IC50 ranging from 6 μM-16 μM and produces its ability to completely reverse the resistance of the oncolytics to the MDR cell lines P388/ADR, MCF7/ADR, 2780AD, or UCLA-P3.003VLB at concentration of 0.1 and 0.5 μM. LY335979 significantly restores drug sensitivity in P-gp-expressing leukemia cell lines including K562/HHT40, K562/HHT90, K562/DOX and HL60/DNR, and enhances the cytotoxicity of anthracyclines and ozogamicin (Mylotarg) in primary AML blasts with active P-gp. A latest paper indicates that LY335979 completely inhibits apically directed transport of (Z)-endoxifen in the ABCB1-transduced cells. |
Protocol (from reference)
| Kinase Assay: |
|
|---|---|
| Cell Assay: |
|
| Animal Study: |
|
References
|
Customer Product Validation
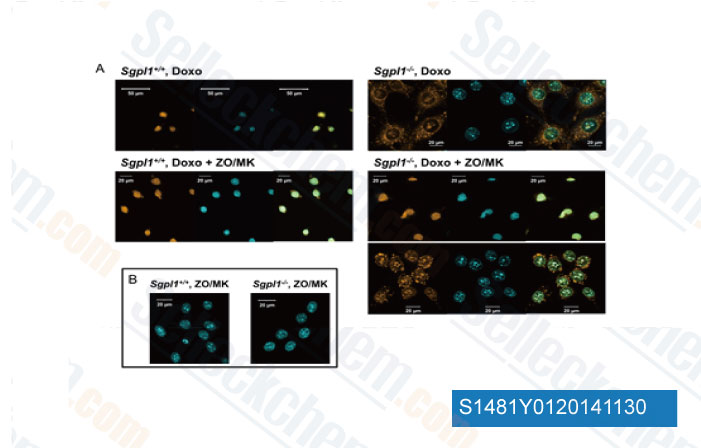
-
Data from [ J Lipid Res , 2014 , 10.1194/jlr.M052761 ]
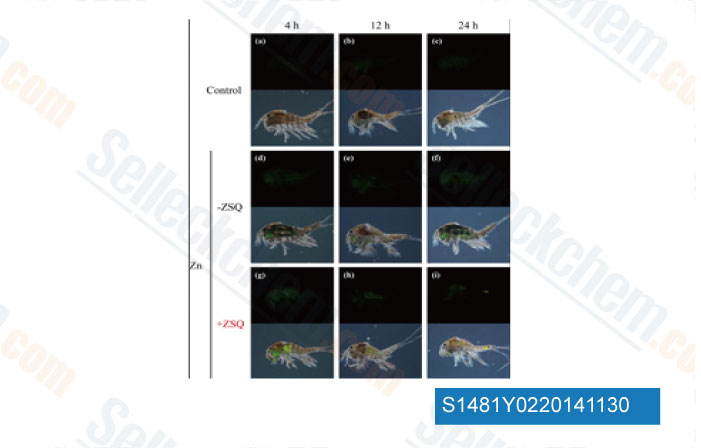
-
Data from [ Aquat Toxicol , 2014 , 156C, 135-147 ]
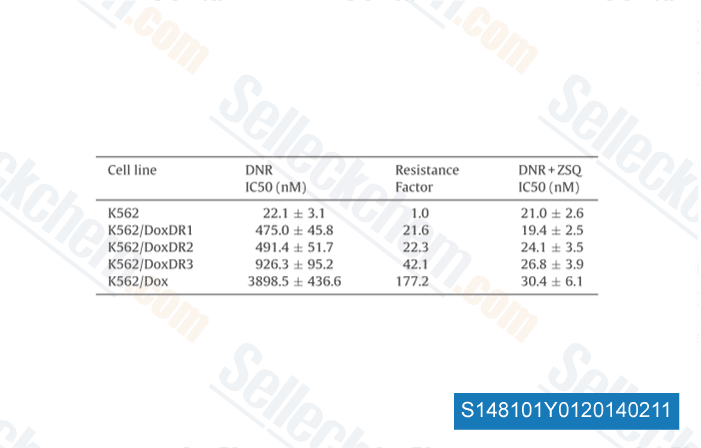
-
Data from [ Pharmacol Res , 2013 , 67(1), 79-83 ]
![Inhibition of B-A steady state fluxes of digoxin and rhodamine 123 by increasing concentrations of zosuquidar (ZSQ). The iP-gp cell monolayers were investigated for digoxin (A) and rhodamine 123 (B) transport in the presence of increasing concentrations of zosuquidar (0.002–2.0 μM). The cells were preincubated with zosuquidar 10 min before addition of 3.12 μM rhodamine 123 or tracer amount of [3H]-digoxin at the abluminal site for B-A transport in a period of 120 min. The fluxes were estimated from the linear part of the accumulation curves. Values are presented as means ± SEM of three individual cell passages with two individual permeable supports (n =3, total N = 6).](https://file.selleckchem.com/downloads/review/700px/Zosuquidar-S148101X0620181107.gif)
-
Data from [ , , Eur J Pharm Sci, 2018, 112:112-121 ]
Selleck's Zosuquidar 3HCl Has Been Cited by 34 Publications
| Development of PROTACs for targeted degradation of oncogenic TRK fusions [ bioRxiv, 2025, 2025.06.18.660465] | PubMed: 40666929 |
| Inhibition of the transmembrane transporter ABCB1 overcomes resistance to doxorubicin in patient-derived organoid models of HCC [ Hepatol Commun, 2024, 8(5)e0437] | PubMed: 38696353 |
| Functional Blood-Brain Barrier Model with Tight Connected Minitissue by Liquid Substrates Culture [ Adv Healthc Mater, 2022, e2201984] | PubMed: 36394091 |
| Overcoming Resistance to Anti–Nectin-4 Antibody-Drug Conjugate [ Mol Cancer Ther, 2022, 21 (7): 1227–1235.] | PubMed: None |
| Overcoming Resistance to Anti-nectin-4 Antibody-Drug Conjugate [ Mol Cancer Ther, 2022, molcanther.0013.2022] | PubMed: 35534238 |
| Brain endothelial cells metabolize glutamate via glutamate dehydrogenase to replenish TCA-intermediates and produce ATP under hypoglycemic conditions [ J Neurochem, 2021, 157(6):1861-1875] | PubMed: 33025588 |
| Heat Shock Protein Inhibitor 17-Allyamino-17-Demethoxygeldanamycin, a Potent Inductor of Apoptosis in Human Glioma Tumor Cell Lines, Is a Weak Substrate for ABCB1 and ABCG2 Transporters [ Pharmaceuticals (Basel), 2021, 14(2)107] | PubMed: 33573093 |
| The blood-brain barrier studied in vitro across species [ PLoS One, 2021, 16(3):e0236770] | PubMed: 33711041 |
| Characterization of human pregnane X receptor activators identified from a screening of the Tox21 compound library [ Biochem Pharmacol, 2020, S0006-2952(20)30604-3] | PubMed: 33333074 |
| Testing of the Survivin Suppressant YM155 in a Large Panel of Drug-Resistant Neuroblastoma Cell Lines. [ Cancers (Basel), 2020, 2;12(3) pii: E577] | PubMed: 32131402 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
