|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C21H20N4 |
||||||||||||||
| Molecular Weight | 328.41 | CAS No. | 881202-45-5 | ||||||||||||
| Solubility (25°C)* | In vitro | DMSO | 66 mg/mL (200.96 mM) | ||||||||||||
| Ethanol | 2 mg/mL (6.08 mM) | ||||||||||||||
| Water | Insoluble | ||||||||||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||||||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||||||||||
Preparing Stock Solutions
Biological Activity
| Description | Serdemetan (JNJ-26854165) acts as a HDM2 ubiquitin ligase antagonist and also induces early apoptosis in p53 wild-type cells, inhibits cellular proliferation followed by delayed apoptosis in the absence of functional p53. Phase 1. | |||
|---|---|---|---|---|
| Targets |
|
|||
| In vitro | JNJ 26854165 is a novel tryptamine derivative which activates p53 and acts as a HDM2 ubiquitin ligase antagonist. JNJ 26854165 inhibits cell growth and induces apoptosis in leukemia cell lines with IC50 values of 0.24, 0.33, 0.32 and 0.44 μM at 72 hours for OCI-AML-3, MOLM-13, NALM-6 and REH cells, respectively. In addition, JNJ 26854165 accelerates proteasome-mediated degradation of p21 and antagonizes the transcriptional induction of p21 by p53. It also induces S-phase delay and upregulates E2F1 expression in p53 mutant cells, resulting in preferential apoptosis of S-phase cells. [1] JNJ 26854165 is an oral Mdm2 inhibitor which can inhibit the interaction of Mdm2-p53 complex with the proteasome and increase p53 levels by binding to RING domain of Mdm2. [2] A recent study shows that JNJ 26854165 inhibits clonogenic survival in four human cancer cell lines: H460, A549, p53-WT-HCT116, and p53-null-HCT116. [3] | |||
| In vivo | JNJ 26854165 leads to significant differences in EFS distribution in 17 of the 36 (47%) evaluable solid tumor xenografts and in 5 of 7 (71%) of the evaluable ALL xenografts using a dose of 20 mg/kg administered via oral gavage daily for 5 days, repeated for 6 weeks. [4] |
Protocol (from reference)
| Cell Assay:[1] |
|
|---|---|
| Animal Study:[4] |
|
References
|
Customer Product Validation
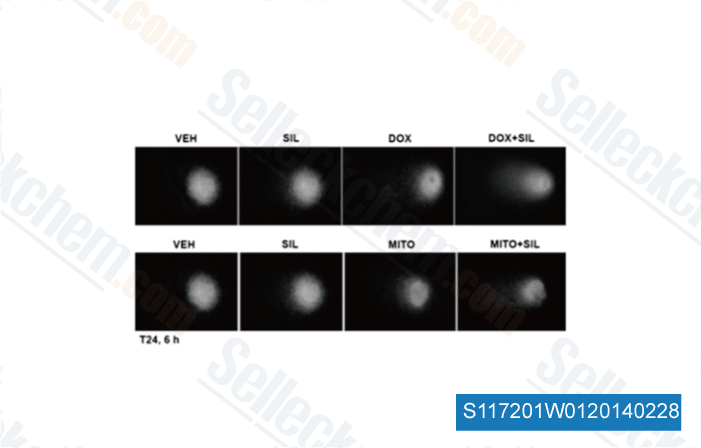
-
Data from [ Mol Pharmacol , 2014 , 85(3), 408-19 ]
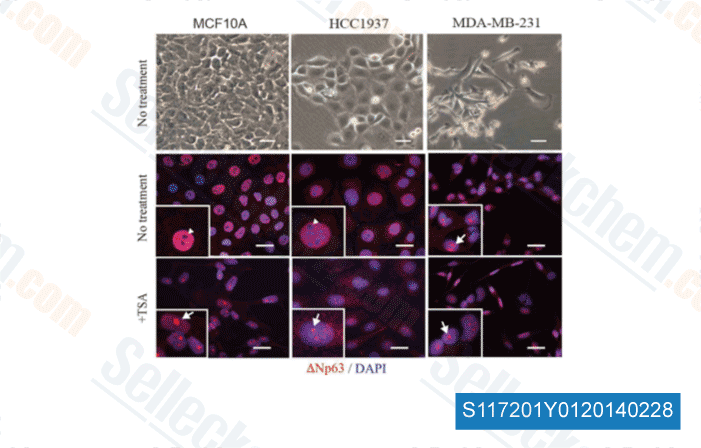
-
Data from [ Sci Rep , 2014 , 4, 4663 ]
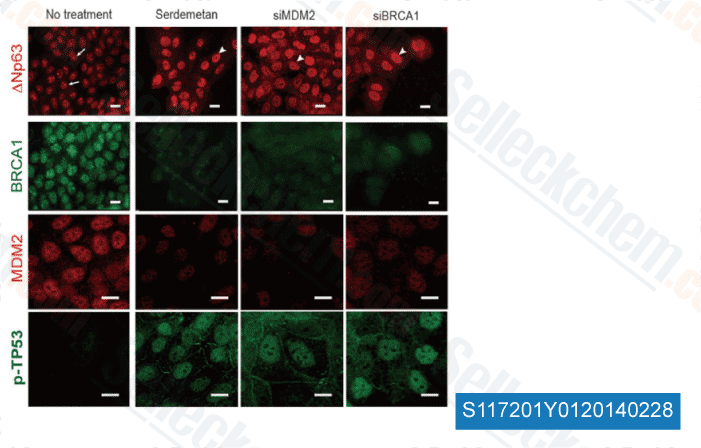
-
Data from [ Sci Rep , 2014 , 4, 4663 ]
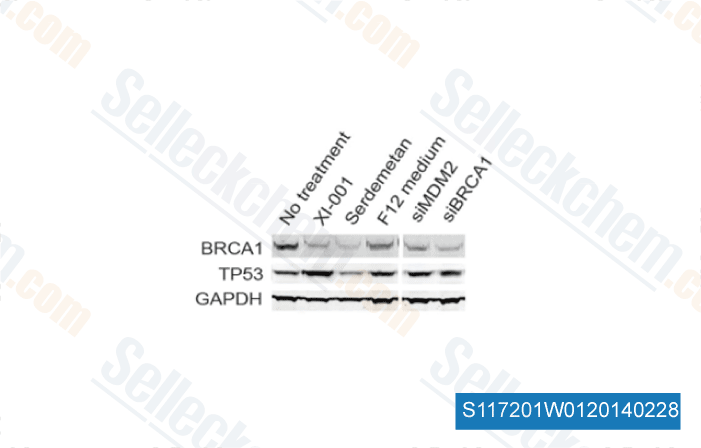
-
Data from [ Sci Rep , 2014 , 4, 4663 ]
Selleck's Serdemetan (JNJ-26854165) Has Been Cited by 15 Publications
| Oversized cells activate global proteasome-mediated protein degradation to maintain cell size homeostasis [ Elife, 2025, 14e75393] | PubMed: 39791360 |
| Endurance training increases a ubiquitylated form of histone H3 in the skeletal muscle, supporting Notch1 upregulation in an MDM2-dependent manner [ J Physiol, 2025, 603(19):5477-5508] | PubMed: 40911795 |
| Nuclear Aurora kinase A switches m6A reader YTHDC1 to enhance an oncogenic RNA splicing of tumor suppressor RBM4 [ Signal Transduct Target Ther, 2022, 7(1):97] | PubMed: 35361747 |
| Prediction and identification of synergistic compound combinations against pancreatic cancer cells [ iScience, 2021, 24(9):103080] | PubMed: 34585118 |
| MDM2-Mediated Ubiquitination of ACE2 Contributes to the Development of Pulmonary Arterial Hypertension [ Circulation, 2020, 10.1161/CIRCULATIONAHA.120.048191] | PubMed: 32755395 |
| Subcellular distribution of p53 by the p53-responsive lncRNA NBAT1 determines chemotherapeutic response in neuroblastoma [ Cancer Res, 2020, canres.3499.2019] | PubMed: 33372039 |
| Gene Essentiality Profiling Reveals Gene Networks and Synthetic Lethal Interactions with Oncogenic Ras. [ Cell, 2019, 36(2):179-193] | PubMed: 28162770 |
| A Pharmacogenomic Landscape in Human Liver Cancers. [ Cancer Cell, 2019, 36(2):179-193] | PubMed: 31378681 |
| The novel anticancer agent JNJ-26854165 is active in chronic myeloid leukemic cells with unmutated BCR/ABL and T315I mutant BCR/ABL through promoting proteosomal degradation of BCR/ABL proteins [ Oncotarget, 2017, 8(5):7777-7790] | PubMed: 27999193 |
| Inhibitors of ubiquitin E3 ligase as potential new antimalarial drug leads. [Jain J, et al. BMC Pharmacol Toxicol, 2017, 18(1):40] | PubMed: 28577368 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
