|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C29H31N7O |
||||||||||
| Molecular Weight | 493.6 | CAS No. | 152459-95-5 | ||||||||
| Solubility (25°C)* | In vitro | DMSO | 23 mg/mL (46.59 mM) | ||||||||
| Water | Insoluble | ||||||||||
| Ethanol | Insoluble | ||||||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||||||
Preparing Stock Solutions
Biological Activity
| Description | Imatinib is a multi-target inhibitor of tyrosine kinase with inhibition for v-Abl, c-Kit and PDGFR; IC50 values are 0.6 μM, 0.1 μM and 0.1 μM in cell-free or cell-based assays, respectively. This compound induces autophagy. | ||||||
|---|---|---|---|---|---|---|---|
| Targets |
|
||||||
| In vitro | In vitro assays for inhibition of a panel of tyrosine and serine/threonine protein kinases show that Imatinib inhibits the v-Abl tyrosine kinase and PDGFR potently with an IC50 of 0.6 and 0.1 μM, respectively. [1] This compound inhibits the SLF-dependent activation of wild-type c-kit kinase activity with a IC50 for these effects of approximately 0.1 μM, which is similar to the concentration required for inhibition of PDGFR. [2] It exhibits growth-inhibitory activity on the human bronchial carcinoid cell line NCI-H727 and the human pancreatic carcinoid cell line BON-1 with an IC50 of 32.4 and 32.8 μM, respectively. [3] A recent study shows that this chemical has the potential to exert its antileukemia effects in chronic myelogenous leukemia by down-regulating hERG1 K(+) channels, which are highly expressed in leukemia cells and appear of exceptional importance in favoring leukemogenesis. [4] |
||||||
| In vivo | Imatinib produces a different antitumor effect on three xenografted tumors derived from surgical samples of fresh human small cell lung cancers, with 80%, 40% and 78% growth inhibition of SCLC6, SCLC61 and SCLC108 tumors, respectively, and no significant inhibition of SCLC74 growth. [5] In high fat fed ApoE(-/-) mice, this compound significantly reduces the high fat-induced lipid staining area by 30%, 27% and 35% compared to high-fat diet untreated controls when dosed by gavage at 10, 20 and 40 mg/kg, respectively, and suppresses carotid artery lipid accumulation. [6] |
Protocol (from reference)
| Kinase Assay: |
|
|---|---|
| Cell Assay: |
|
References
|
Customer Product Validation
![<p> </p><p>Inhibition of thymidine (a and b) and cytarabine (c and d) uptake with imatinib. K562 cells (a and c) and MEG-01 cells (b and d) were incubated at 37 ◦C for 15 min with imatinib transport buffer, and then incubated with 0.5 Ci of [3H] thymidine or [3H] cytarabine for an additional 5 min in presence of imatinib. Cells were then washed 3 times, lysed and radioactivity associated to cell pellets was quantified. DMSO, dimethylsulfoxide; DPD, dipyridamole.</p>](https://file.selleckchem.com/downloads/review/700px/Imatinib-S247501Z0120130501.gif)
-
Data from [ Leukemia Res , 2012 , 36, 1311-1314 ]
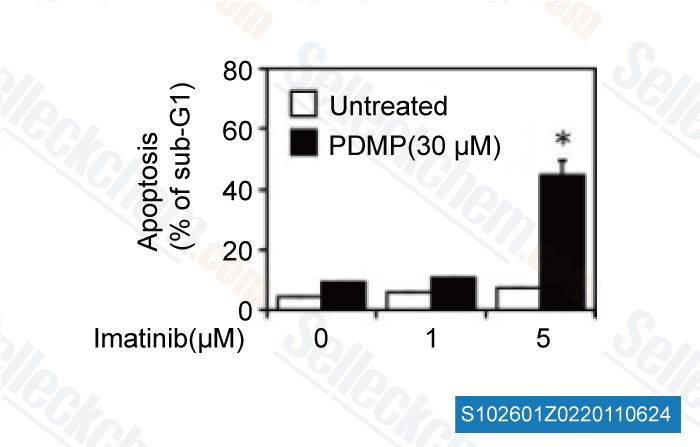
-
Data from [ FASEB J , 2011 , 25, 3661-3673 ]
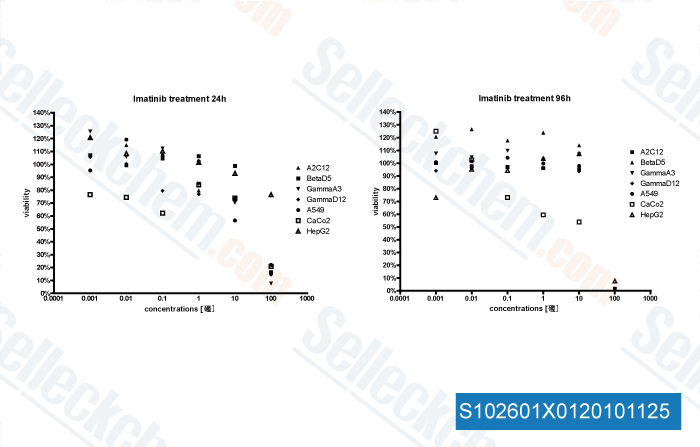
-
, , Dr. Thomas Kruwel of Fraunhofer
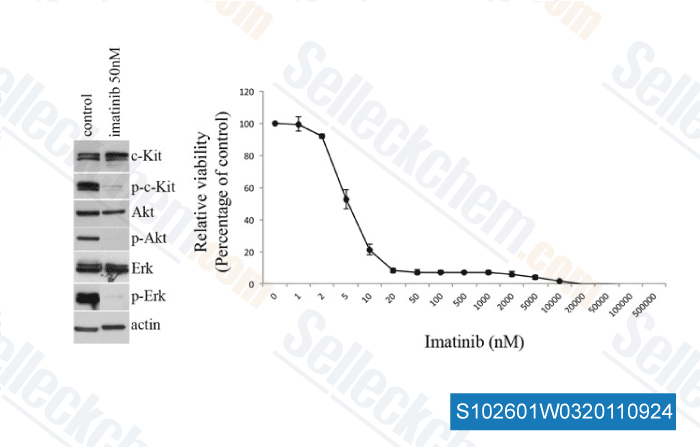
-
, , Dr. Helen Rizos from the university of Sydney
Selleck's Imatinib Has Been Cited by 338 Publications
| Proteogenomic characterization of non-functional pancreatic neuroendocrine tumors unravels clinically relevant subgroups [ Cancer Cell, 2025, 43(4):776-796.e14] | PubMed: 40185092 |
| CD19-targeted HSP90 inhibitor nanoparticle combined with TKIs reduces tumor burden and enhances T-cell immunity in murine B-cell malignancies [ Theranostics, 2025, 15(8):3589-3609] | PubMed: 40093890 |
| The landcape of Helicobacter pylori-mediated DNA breaks links bacterial genotoxicity to its oncogenic potential [ Genome Med, 2025, 17(1):14] | PubMed: 39994739 |
| Haploinsufficiency of miR-143 and miR-145 reveal targetable dependencies in resistant del(5q) myelodysplastic neoplasm [ Leukemia, 2025, 39(4):917-928] | PubMed: 40000845 |
| Systematic analysis of ZDHHC9 as a potential prognostic and immunotherapy biomarker in breast cancer [ Front Immunol, 2025, 16:1609621] | PubMed: 40740766 |
| Methylstat sensitizes ovarian cancer cells to PARP-inhibition by targeting the histone demethylases JMJD1B/C [ Cancer Gene Ther, 2025, 10.1038/s41417-025-00874-z] | PubMed: 39915607 |
| Activation of TMEM16E scramblase induces ligand independent growth factor receptor signaling and macropinocytosis for membrane repair [ Commun Biol, 2025, 8(1):35] | PubMed: 39794444 |
| Characterization of the Temporal Dynamics of the Endothelial-Mesenchymal-like Transition Induced by Soluble Factors from Dengue Virus Infection in Microvascular Endothelial Cells [ Int J Mol Sci, 2025, 26(5)2139] | PubMed: 40076764 |
| Utility of the Base Editing System for Introducing Drug-Resistant Gene Mutations Into Human Leukemia Cellular Models [ Cureus, 2025, 17(4):e81889] | PubMed: 40342439 |
| Genome-wide Cas9-mediated screening of essential non-coding regulatory elements via libraries of paired single-guide RNAs [ Nat Biomed Eng, 2024, 10.1038/s41551-024-01204-8] | PubMed: 38778183 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
