|
How to Cite 1. For In-Text Citation (Materials & Methods): 2. For Key Resources Table: |
||
|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. We strive to reply to |
Technical Data
| Formula | C27H37N3O7S.C2H5OH |
||||||
| Molecular Weight | 593.73 | CAS No. | 635728-49-3 | ||||
| Solubility (25°C)* | In vitro | DMSO | 100 mg/mL (168.42 mM) | ||||
| Water | Insoluble | ||||||
| Ethanol | Insoluble | ||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||
Preparing Stock Solutions
Biological Activity
| Description | Darunavir Ethanolate (TMC-114, UIC 94017) is a nonpeptidic HIV protease inhibitor, used to treat HIV infection. | |
|---|---|---|
| Targets |
|
|
| In vitro | Darunavir displays potent activity against HIV strains resistant to other available protease inhibitor. Darunavir inhibits P-glycoprotein-mediated efflux of calcein-acetoxymethyl ester in L-MDR1 cells with the inhibitory potency of 121 mM. Darunavir is a protein inhibits that mimics the phenylalanine sequences at positions 167 and 168 of the gag-pol polypeptide and binds to the active sites of the HIV protease, thereby inhibiting its activity. Darunavir blocks the infectivity and replication of each of the HIV-1 variants at concentrations up to 5 μM. Darunavir shows strong ARV activity against a selected panel of 19 recombinant clinical isolates carrying multiple protease mutations conferring resistance to an average of five other protien inhibitors. Darunavir inhibits 75% of 1501 PI-resistant viruses tested with a half maximal effective concentration (EC50) of < 10 nM. |
Protocol (from reference)
References
|
Customer Product Validation
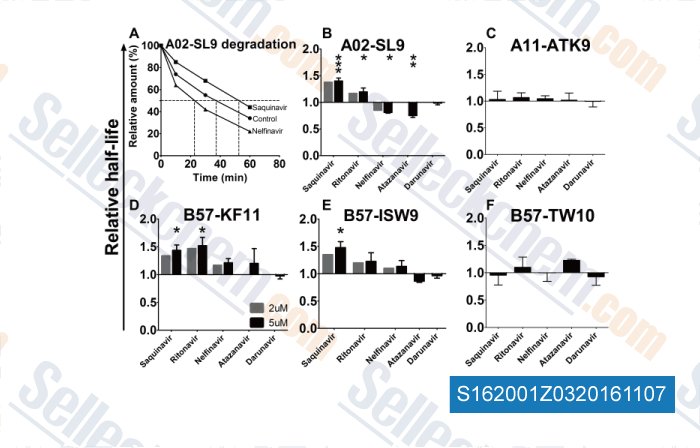
-
, , Drug Metab Dispos, 2016, 44(3):398-408.
Selleck's Darunavir Ethanolate Has Been Cited by 8 Publications
| Reduction of CD8 T cell functionality but not inhibitory capacity by integrase inhibitors [ J Virol, 2022, JVI0173021] | PubMed: 35019724 |
| Integrative multiomics and in silico analysis revealed the role of ARHGEF1 and its screened antagonist in mild and severe COVID-19 patients [ J Cell Biochem, 2022, 10.1002/jcb.30213] | PubMed: 35037717 |
| Characterization of fluorescent probe substrates to develop an efficient high-throughput assay for neonatal hepatic CYP3A7 inhibition screening [ Sci Rep, 2021, 11(1):19443] | PubMed: 34593846 |
| Discovery of M Protease inhibitors encoded by SARS-CoV-2 [ Antimicrob Agents Chemother, 2020, AAC.00872-20] | PubMed: 32669265 |
| Effect of HIV infection and antiretroviral therapy on immune cellular functions. [ JCI Insight, 2019, 4(12)] | PubMed: 31217351 |
| HIV-1 Subtype C with PYxE Insertion Has Enhanced Binding of Gag-p6 to Host Cell Protein ALIX and Increased Replication Fitness. [ J Virol, 2019, 93(9)] | PubMed: 30760577 |
| Solitary inhibition of the breast cancer resistance protein (BCRP) efflux transporter results in a clinically significant drug-drug interaction with rosuvastatin by causing up to a two-fold increase in statin exposure [Elsby R, et al. Drug Metab Dispos, 2015, 44(3):398-408] | PubMed: 26700956 |
| Sequence-Specific Alterations of Epitope Production by HIV Protease Inhibitors. [Kourjian G, et al. J Immunol, 2014, 192(8):3496-506] | PubMed: 24616479 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
