|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C9H13N3O5 |
||||||
| Molecular Weight | 243.22 | CAS No. | 147-94-4 | ||||
| Solubility (25°C)* | In vitro | DMSO | 48 mg/mL (197.35 mM) | ||||
| Water | 48 mg/mL (197.35 mM) | ||||||
| Ethanol | Insoluble | ||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||
Preparing Stock Solutions
Biological Activity
| Description | Cytarabine is an antimetabolic agent and DNA synthesis inhibitor with IC50 of 16 nM in wild-type CCRF-CEM cells. Cytarabine induces autophagy and apoptosis. | ||
|---|---|---|---|
| Targets |
|
||
| In vitro | Cytarabine (AraC) is phosphorylated into a triphosphate form (Ara-CTP) involving deoxycytidine kinase (dCK), which competes with dCTP for incorporation into DNA, and then blocks DNA synthesis by inhibiting the function of DNA and RNA polymerases. Cytarabine displays a higher growth inhibitory activity towards wild-type CCRF-CEM cells compared to other acute myelogenous leukemia (AML) cells with IC50 of 16 nM. [1] Increasing concentrations of Cytarabine (IC50 of 0.69 μM) results in decreased metabolic activity of sensitive rat leukemic cell line RO/1, and the cell toxity can be highly enhanced by transfection with human wt dCK (IC50 of 0.037 μM) but not the inactive, alternatively spliced dCK forms. [2] Cytarabine apparently induces apoptosis of rat sympathetic neurons at 10 μM, of which 100 μM shows the highest toxicity and kills over 80% of the neurons by 84 hours, involving the release of mitochondrial cytochrome-c and the activation of caspase-3, and the toxicity can be attenuated by p53 knockdown and delayed by bax deletion. [3] |
||
| In vivo | Cytarabine is highly effective against acute leukaemias, which causes the characteristic G1/S blockage and synchronization, and increases the survival time for leukaemic Brown Norway rats in a weak dose-related fashion indicating that the use of higher dosages of Cytarabine does not contribute to its antileukaemic effectiveness in man. [4] Cytarabine (250 mg/kg) also causes placental growth retardation and increases placental trophoblastic cells apoptosis in the placental labyrinth zone of the pregnant Slc:Wistar rats, which increases from 3 hour after the treatment and peaks at 6 hour before returning to control levels at 48 hour, with remarkably enhanced p53 protein, p53 trancriptional target genes such as p21, cyclinG1 and fas and caspase-3 activity. [5] |
||
| Features | The 1st of a series of cancer drugs that alters the sugar component of nucleosides. |
Protocol (from reference)
| Kinase Assay: |
|
|---|---|
| Cell Assay: |
|
| Animal Study: [4] |
|
References
|
Customer Product Validation
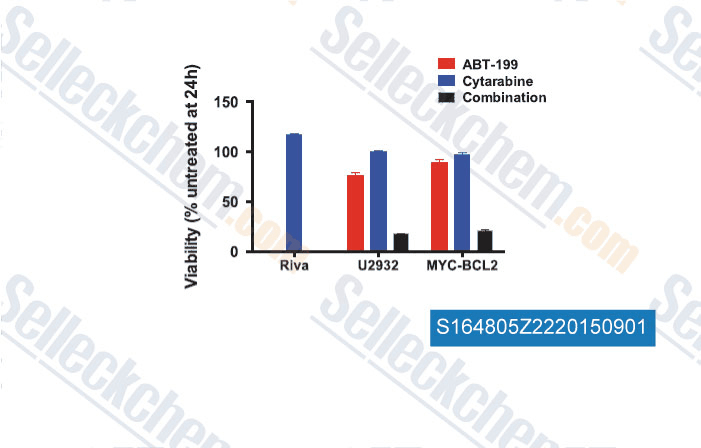
-
Data from [ , , Leukemia, 2015, 29(8): 1702–1712 ]
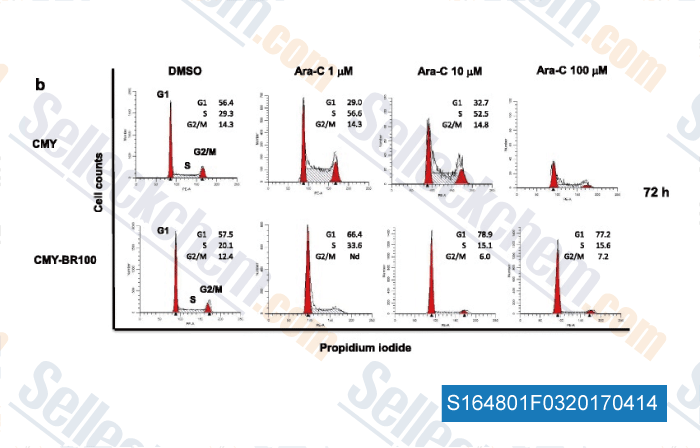
-
Data from [ , , J Exp Clin Cancer Res, 2017, 36(1):22 ]
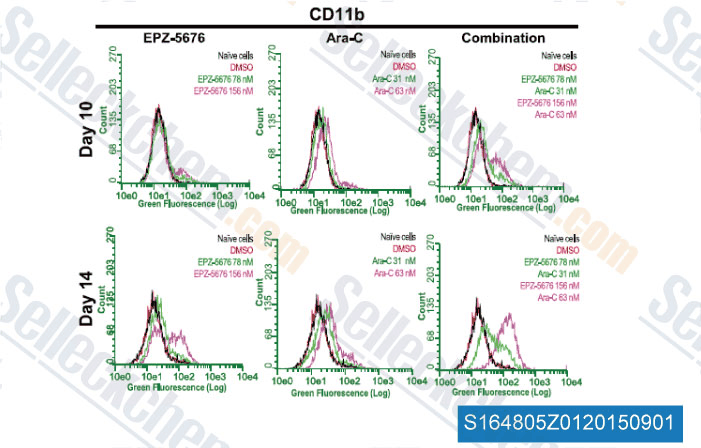
-
Data from [ , , J Pharmacol Exp Ther, 2014, 350(3): 646-56 ]
Selleck's Cytarabine Has Been Cited by 122 Publications
| The ESCRT protein CHMP5 promotes T cell leukemia by enabling BRD4-p300-dependent transcription [ Nat Commun, 2025, 16(1):4133] | PubMed: 40319015 |
| Chidamide and cytarabine synergistically treat acute myeloid leukemia: inhibiting ribosome biogenesis via the MYC-RRP9 pathway [ Cell Death Dis, 2025, 16(1):601] | PubMed: 40781078 |
| The ADCY1-mediated cAMP signaling pathway mediates functional effects of montelukast treatment in brain organoids [ Cell Mol Life Sci, 2025, 82(1):224] | PubMed: 40471331 |
| The ADCY1-mediated cAMP signaling pathway mediates functional effects of montelukast treatment in brain organoids [ Cell Mol Life Sci, 2025, 82(1):224] | PubMed: 40471331 |
| Genome-wide CRISPR/Cas9 screen identifies AraC-daunorubicin-etoposide response modulators associated with outcomes in pediatric AML [ Blood Adv, 2025, 9(5):1078-1091] | PubMed: 39715471 |
| Mitochondrial dysfunction fuels drug resistance in adult T-cell acute lymphoblastic leukemia [ J Transl Med, 2025, 23(1):542] | PubMed: 40369632 |
| Establishment of a prognostic model based on ER stress-related cell death genes and proposing a novel combination therapy in acute myeloid leukemia [ J Transl Med, 2025, 23(1):566] | PubMed: 40399990 |
| Disulfidptosis in pediatric AML: a multi-omics approach to risk stratification and potential therapeutic strategy [ Cancer Cell Int, 2025, 25(1):199] | PubMed: 40457386 |
| Iron overload mediates cytarabine resistance in AML by inhibiting the TP53 signaling pathway [ Acta Biochim Biophys Sin (Shanghai), 2025, 57(4):646-655] | PubMed: 40230093 |
| Integrative Genomic and Immune Profiling to Identify and Characterize High-Risk Subgroups in Acute Myeloid Leukemia: Development of a 20-Gene Predictive Signature and Its Clinical Implications [ OMICS, 2025, 29(9):458-472] | PubMed: 40844427 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
