|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. |
Technical Data
| Formula | C16H20N6O.C6H8O7 |
|||
| Molecular Weight | 504.49 | CAS No. | 540737-29-9 | |
| Solubility (25°C)* | In vitro | DMSO | 100 mg/mL warmed with 50ºC water bath (198.21 mM) | |
| Water | Insoluble | |||
| Ethanol | Insoluble | |||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
||||
Preparing Stock Solutions
Biological Activity
| Description | Tofacitinib citrate (CP-690550, Tasocitinib) is a novel inhibitor of JAK with IC50 of 1 nM, 20 nM and 112 nM against JAK3, JAK2, and JAK1, respectively. Tofacitinib citrate has anti-infection activity. | ||||
|---|---|---|---|---|---|
| Targets |
|
||||
| In vitro | Tofacitinib citrate inhibits IL-2-mediated human T cell blast proliferation and IL-15-induced CD69 expression with IC50 of 11 nM and 48 nM, respectively. Tofacitinib citrate prevents mixed lymphocyte reaction with IC50 of 87 nM. Tofacitinib citrate treatment of murine factor-dependent cell Patersen–erythropoietin receptor (FDCP-EpoR) cells harboring human wild-type or V617F JAK2 leads to prevention of cell proliferation with IC50 of 2.1 µM and 0.25 µM, respectively. Tofacitinib citrate inhibits interleukin-6-induced phosphorylation of STAT1 and STAT3 with IC50 of 23 nM and 77 nM, respectively. Moreover, Tofacitinib citrate generates a significant pro-apoptotic effect on murine FDCP-EpoR cells carrying JAK2VV617F, whereas a lesser effect is observed for cells carrying wild-type JAK2. This activity is coupled with the inhibition of phosphorylation of the key JAK2V617F-dependent downstream signaling effectors signal transducer and activator of transcription (STAT)3, STAT5, and v-akt murine thymoma viral oncogene homolog (AKT). [2] Additionally, Tofacitinib citrate prevents IL-15-induced CD69 expression in human and cynomolgus monkey NK and CD8+ T cells in vitro. [3] | ||||
| In vivo | Tofacitinib citrate decrease a delayed-type hyper-sensitivity response and extended cardiac allograft survival in murine models. Furthermore, Tofacitinib citrate treatment of ex-vivo-expanded erythroid progenitors from JAK2V617F-positive PV patients results in specific, antiproliferative (IC50 = 0.2 μM) and pro-apoptotic activity. In contrast, expanded progenitors from healthy controls are less sensitive to Tofacitinib citrate in proliferation (IC50 > 1.0 μM), and apoptosis assays.[2] During 2 weeks of Tofacitinib citrate dosing at 10 and 30 mg/kg/d, a significant, time-dependent decrease in NK cell numbers relative to vehicle treatment is observed. Effector memory CD8+ cell numbers in the Tofacitinib citrate-treated group are 55% less than those observed in animals treated with vehicle.[3] |
Protocol (from reference)
| Kinase Assay: |
|
|---|---|
| Cell Assay: |
|
| Animal Study: |
|
References
|
Customer Product Validation
-
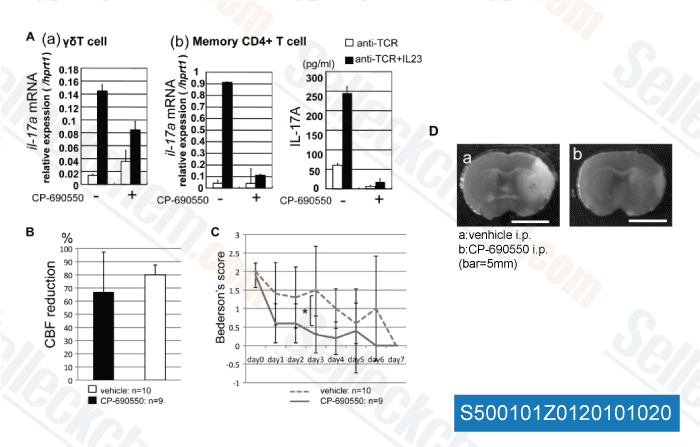
Data from [ Biochem Biophy Res Commun , 2010 , 402, 500–506 ]
-
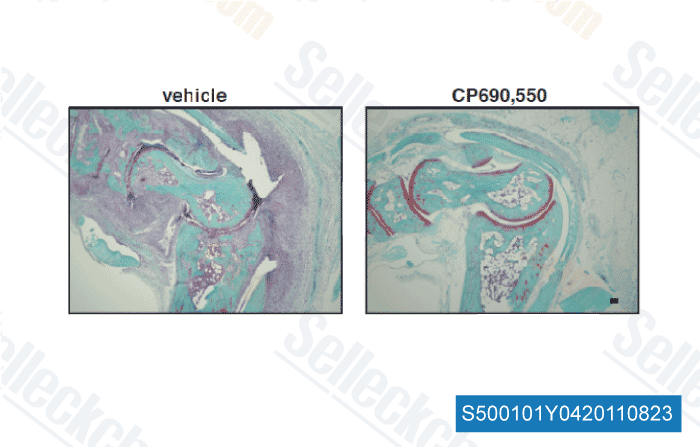
, , Dr. Akihiko Yoshimura of Akihiko Yoshimura
-
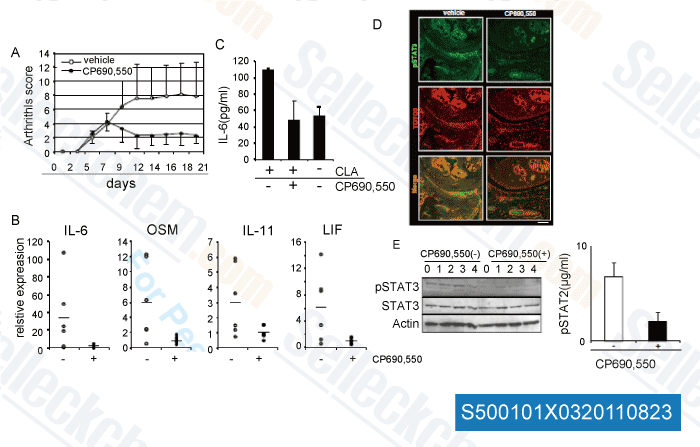
, , Saraswati Sukumar of Johns Hopkins University School of Medicine
-
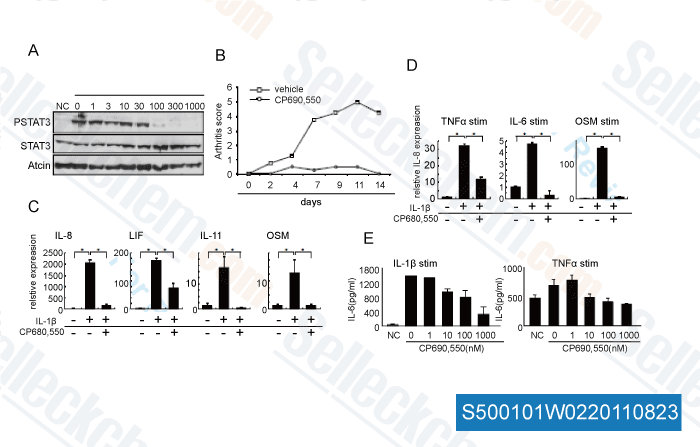
, , Dr. Akihiko Yoshimura of Akihiko Yoshimura
Selleck's Tofacitinib (CP-690550) Citrate Has Been Cited by 220 Publications
| Unveiling the Anti-Angiogenic Potential of Small-Molecule (Kinase) Inhibitors for Application in Rheumatoid Arthritis [ Cells, 2025, 14(2)102] | PubMed: 39851530 |
| Pharmacological evaluation of drug therapies in Aicardi-Goutières syndrome: insights from patient-derived neural stem cells [ Front Pharmacol, 2025, 16:1549183] | PubMed: 40183101 |
| JAK/STAT3 represents a therapeutic target for colorectal cancer patients with stromal-rich tumors [ J Exp Clin Cancer Res, 2024, 43(1):64] | PubMed: 38424636 |
| Patient-derived rhabdomyosarcoma cells recapitulate the genetic and transcriptomic landscapes of primary tumors [ iScience, 2024, 27(10):110862] | PubMed: 39319271 |
| A panel of janus kinase inhibitors identified with anti-inflammatory effects protect mice from lethal influenza virus infection [ Antimicrob Agents Chemother, 2024, 68(4):e0135023.] | PubMed: 38470034 |
| Tofacitinib to prevent anti-drug antibody formation against LMB-100 immunotoxin in patients with advanced mesothelin-expressing cancers [ Front Oncol, 2024, 14:1386190] | PubMed: 38706610 |
| Tofacitinib to prevent anti-drug antibody formation against LMB-100 immunotoxin in patients with advanced mesothelin-expressing cancers [ Front Oncol, 2024, 14:1386190] | PubMed: 38706610 |
| Effect of JAK inhibitors on the three forms of bone damage in autoimmune arthritis: joint erosion, periarticular osteopenia, and systemic bone loss [ Inflamm Regen, 2023, 43(1):44] | PubMed: 37726797 |
| Metabolic signature and proteasome activity controls synovial migration of CDC42hiCD14+ cells in rheumatoid arthritis [ Front Immunol, 2023, 14:1187093] | PubMed: 37662900 |
| Comprehensive protocols for culturing and molecular biological analysis of IBD patient-derived colon epithelial organoids [ Front Immunol, 2023, 14:1097383] | PubMed: 36911731 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
