|
How to Cite 1. For In-Text Citation (Materials & Methods): 2. For Key Resources Table: |
||
|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. We strive to reply to |
Technical Data
| Formula | C6H12O5 |
||||||
| Molecular Weight | 164.16 | CAS No. | 154-17-6 | ||||
| Solubility (25°C)* | In vitro | Water | 100 mg/mL (609.16 mM) | ||||
| DMSO | 33 mg/mL (201.02 mM) | ||||||
| Ethanol | 5 mg/mL (30.45 mM) | ||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||
Preparing Stock Solutions
Biological Activity
| Description | 2-DG (2-Deoxy-D-glucose), an analog of glucose, is a glycolytic inhibitor with antiviral activity. 2-Deoxy-D-glucose induces apoptosis and inhibits Herpes Simplex Virus type-1 (HSV-1) receptor expression. | |
|---|---|---|
| Targets |
|
|
| In vitro | 2-Deoxy-D-glucose (2-DG) activates AKT function through phosphatidylinositol 3-kinase (PI3K) and is independent of glycolysis or mTOR inhibition. Its treatments disrupt the binding between insulin-like growth factor 1 (IGF-1) and IGF-binding protein 3 (IGFBP3) so that the free form of IGF-1 could be released from the IGF-1·IGFBP3 complex to activate IGF-1 receptor (IGF1R) signaling. 2-DG-induced activation of many survival pathways can be jointly attenuated through IGF1R inhibition. This compound also induces time- and dose-dependent ERK phosphorylation. It is readily transported into cells and is phosphorylated by hexokinase, but cannot be metabolized further and accumulates in the cell. This leads to ATP depletion and the induction of cell-death. 2DG significantly suppresses proliferation, causes apoptosis and reduces migration of murine endothelial cells, inhibiting formation of lamellipodia and filopodia and causing disorganization of F-actin filaments in murine endothelial cell. | |
| In vivo | Treatment of cancer patients with relatively high doses of 2-Deoxy-D-glucose (2-DG) (greater than 200 mg/kg) was largely ineffective in managing tumor growth. Side effects of this compound included elevated blood glucose levels, progressive weight loss with lethargy, and behavioral symptoms of hypoglycemia. It enhances isoflurane-induced loss of righting reflex in mice. By reducing metabolism, 2-DG treatment can decrease body temperature in rodent, enhancing sensitivity to anesthetics. A diet containing it significantly increased serum ketone body level and brain expression of enzymes required for ketone body metabolism. The 2-DG-induced maintenance of mitochondrial bioenergetics was paralleled by simultaneous reduction in oxidative stress. Further, treated mice exhibited a significant reduction of both amyloid precursor protein (APP) and amyloid beta (Aβ) oligomers, which was paralleled by significantly increased α-secretase and decreased γ-secretase expression, indicating that this compound induced a shift towards a non-amyloidogenic pathway. It increased expression of genes involved in Aβ clearance pathways, degradation, sequestering, and transport. Concomitant with increased bioenergetic capacity and reduced β-amyloid burden, 2-DG significantly increased expression of neurotrophic growth factors, BDNF and NGF, thus reduces pathology in female mouse model of Alzheimer's disease. |
Protocol (from reference)
| Cell Assay: |
|
|---|---|
| Animal Study: |
|
References
|
Customer Product Validation
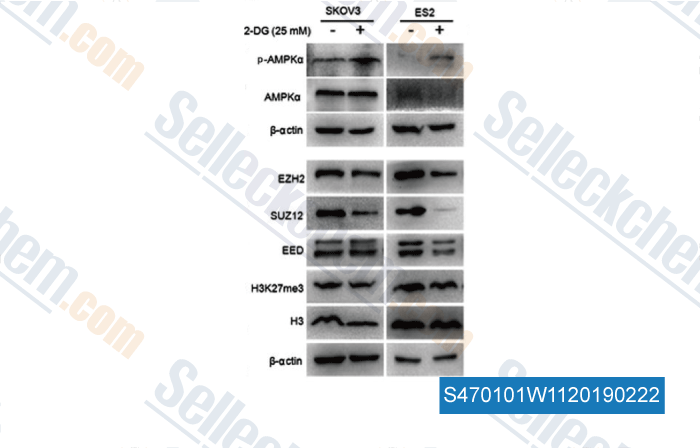
-
Data from [ , , Int J Oncol, 2018, 52(6):1899-1911 ]
Selleck's 2-DG (2-Deoxy-D-glucose) Has Been Cited by 97 Publications
| The noncanonical function of liver-type phosphofructokinase potentiates the efficacy of HDAC inhibitors in cancer [ Signal Transduct Target Ther, 2025, 10(1):341] | PubMed: 41083431 |
| Mitochondrial-cytochrome c oxidase II promotes glutaminolysis to sustain tumor cell survival upon glucose deprivation [ Nat Commun, 2025, 16(1):212] | PubMed: 39747079 |
| Simvastatin overcomes the pPCK1-pLDHA-SPRINGlac axis-mediated ferroptosis and chemo-immunotherapy resistance in AKT-hyperactivated intrahepatic cholangiocarcinoma [ Cancer Commun (Lond), 2025, 10.1002/cac2.70036] | PubMed: 40443016 |
| Aldehyde Dehydrogenase 2 Lactylation Aggravates Mitochondrial Dysfunction by Disrupting PHB2 Mediated Mitophagy in Acute Kidney Injury [ Adv Sci (Weinh), 2025, 12(8):e2411943] | PubMed: 39737891 |
| Energy Deficiency-Induced ATG4B Nuclear Translocation Inhibits PRMT1-Mediated DNA Repair and Promotes Leukemia Progression [ Adv Sci (Weinh), 2025, 12(40):e09838] | PubMed: 40788108 |
| Lactylation of CREB is required for FSH-induced proliferation and differentiation of ovarian granulosa cells [ Nucleic Acids Res, 2025, 53(17)gkaf882] | PubMed: 40966521 |
| A CD147-targeted small-molecule inhibitor potentiates gemcitabine efficacy by triggering ferroptosis in pancreatic ductal adenocarcinoma [ Cell Rep Med, 2025, 6(8):102292] | PubMed: 40834852 |
| PDK4-driven lactate accumulation facilitates LPCAT2 lactylation to exacerbate sepsis-induced acute lung injury [ Cell Death Differ, 2025, 10.1038/s41418-025-01585-6] | PubMed: 41057687 |
| Glycolysis Induces Abnormal Transcription Through Histone Lactylation in T-lineage Acute Lymphoblastic Leukemia [ Genomics Proteomics Bioinformatics, 2025, qzaf029] | PubMed: 40193528 |
| HIF-1-mediated macrophage metabolic reprogramming promotes AKI to CKD transition [ Int J Biol Sci, 2025, 21(13):5936-5955] | PubMed: 41079928 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
