- Bioactive Compounds
- By Signaling Pathways
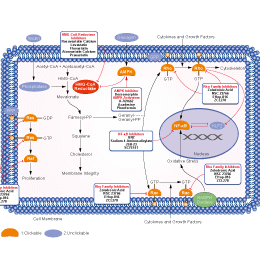
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- Compound Libraries
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-Ⅱ
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Plant Extract Library
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at [email protected] to customize your library.
You could select:
- Antibodies
- Bioreagents
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- Protein Assay
- Protein A/G Magnetic Beads for IP
- Anti-Flag magnetic beads
- Anti-Flag Affinity Gel
- Anti-Myc magnetic beads
- Anti-HA magnetic beads
- Poly FLAG Peptide lyophilized powder
- Protease Inhibitor Cocktail
- Protease Inhibitor Cocktail (EDTA-Free, 100X in DMSO)
- Phosphatase Inhibitor Cocktail (2 Tubes, 100X)
- Cell Biology
- Cell Counting Kit-8 (CCK-8)
- Animal Experiment
- Mouse Direct PCR Kit (For Genotyping)
- New Products
- Contact Us
Lovastatin
Synonyms: Mevinolin, MK-803
Lovastatin is an inhibitor of HMG-CoA reductase with IC50 of 3.4 nM in a cell-free assay, used for lowering cholesterol (hypolipidemic agent). Lovastatin triggers autophagy.
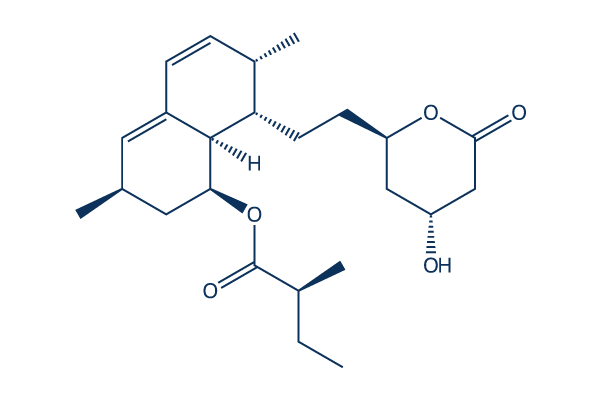
Lovastatin Chemical Structure
CAS: 75330-75-5
Selleck's Lovastatin has been cited by 50 publications
Purity & Quality Control
Batch:
Purity:
99.99%
99.99
Lovastatin Related Products
| Related Products | Simvastatin Fluvastatin Sodium Atorvastatin Pitavastatin calcium Rosuvastatin calcium Atorvastatin Calcium Pravastatin sodium Pravastatin Mevastatin SR-12813 Clinofibrate | Click to Expand |
|---|---|---|
| Related Compound Libraries | Metabolism Compound Library Anti-cancer Metabolism Compound Library Glutamine Metabolism Compound Library Carbohydrate Metabolism Compound Library Lipid Metabolism Compound Library | Click to Expand |
Signaling Pathway
Choose Selective HMG-CoA Reductase Inhibitors
Cell Data
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| HES 9 cell line | Function assay | Concentration required to inhibit HMG-CoA reductase by 50% was determined in HES 9 cell line, IC50=0.013 μM | 1527791 | |||
| HEP G2 | Function assay | Inhibition of the incorporation of sodium [14C]acetate into cholesterol in HEP G2 cells., IC50=0.05μM. | 1656041 | |||
| HEP G2 | Function assay | Inhibition of cellular HMG-CoA reductase in cultures of hepatic cells (HEP G2, a human hepatoma cell line), IC50=0.00005μM. | 2153213 | |||
| HEP G2 | Function assay | Inhibition of cellular HMG-CoA reductase in cultures of human HEP G2 cells, determined by decreased incorporation of sodium [14C]acetate into cholesterol., IC50=0.05μM. | 2296036 | |||
| HEP G2 | Function assay | Tested for inhibition of cholesterol biosynthesis in HEP G2 cells, IC50=0.029μM. | 7932551 | |||
| HEP-G2 | Function assay | Tested for ability to inhibit incorporation of [14C]acetate into cholesterol in cultured human hepatoma (HEP-G2) cells; 0.061-0.10, IC50=0.079μM. | 8246237 | |||
| 3T3-G185 | Function assay | TP_TRANSPORTER: inhibition of Daunorubicin transport in 3T3-G185 cells, IC50=26μM. | 11474784 | |||
| NIH-3T3-G185 | Function assay | TP_TRANSPORTER: inhibition of LDS-751 efflux in NIH-3T3-G185 cells, IC50=32.7μM. | 11716514 | |||
| MDCK | Function assay | TP_TRANSPORTER: inhibition of calcein-AM efflux in MDR1-expressing MDCK cells, IC50=10μM. | 15616150 | |||
| HEK293 | Function assay | TP_TRANSPORTER: inhibition of estradiol-17beta-glucuronide uptake(estradiol-17beta-glucuronide:0.02uM) in OATP1B1-expressing HEK293 cells, IC50=28μM. | 15616150 | |||
| Huh-7/3-1 | Antiviral assay | 72 hrs | Antiviral activity against Hepatitis C virus (isolate Con1) genotype 1b in human Huh-7/3-1 cells assessed as inhibition of HCV replication after 72 hrs by luciferase assay | 16408072 | ||
| SW480 | Growth inhibition assay | 96 hrs | Growth inhibition of human SW480 cells after 96 hrs by MTS assay, IC50=7.1μM. | 17472962 | ||
| LS180 | Growth inhibition assay | 96 hrs | Growth inhibition of human LS180 cells after 96 hrs by MTS assay, IC50=25.3μM. | 17472962 | ||
| HT29 | Growth inhibition assay | 96 hrs | Growth inhibition of human HT29 cells after 96 hrs by MTS assay, IC50=46.8μM. | 17472962 | ||
| SW480 | Growth inhibition assay | 20 uM | 48 hrs | Reversal of growth inhibition of human SW480 cells at 20 uM after 48 hrs by MTS assay in presence of >50 uM mevalonate | 17472962 | |
| SW480 | Function assay | 20 uM | 48 hrs | Decrease in survivin expression in human SW480 cells at 20 uM after 48 hrs by immunoblot analysis | 17472962 | |
| SW480 | Function assay | 20 uM | 48 hrs | Reversal of reduction in survivin expression in human SW480 cells at 20 uM after 48 hrs by immunoblot analysis in presence of 100 uM mevalonate | 17472962 | |
| SW480 | Function assay | 20 uM | 48 hrs | Reversal of reduction in survivin mRNA expression in human SW480 cells at 20 uM after 48 hrs by RT-PCR technique in presence of 100 uM mevalonate | 17472962 | |
| LS180 | Function assay | 20 uM | Inhibition of survivin expression in parent human LS180 cells at 20 uM by immunoblot analysis | 17472962 | ||
| LS180 | Function assay | 20 uM | Inhibition of survivin expression in survivin gene transfected human LS180 cells at 20 uM immunoblot analysis | 17472962 | ||
| SW480 | Growth inhibition assay | 20 uM | 48 hrs | Reversal of growth inhibition of human SW480 cells at 20 uM after 48 hrs by immunoblot analysis in presence of farnesyl pyrophosphate | 17472962 | |
| SW480 | Growth inhibition assay | 20 uM | 48 hrs | Reversal of growth inhibition of human SW480 cells at 20 uM after 48 hrs by immunoblot analysis in presence of geranylgeranyl pyrophosphate | 17472962 | |
| SW480 | Function assay | 20 uM | 48 hrs | Decrease in isoprenylated Ras level in human SW480 cells at 20 uM after 48 hrs by immunoblot analysis | 17472962 | |
| SW480 | Function assay | 20 uM | 48 hrs | Reversal of decrease in isoprenylated Ras level in human SW480 cells at 20 uM after 48 hrs by immunoblot analysis in presence of mevalonate | 17472962 | |
| SW480 | Function assay | 20 uM | 48 hrs | Reversal of decrease in isoprenylated Ras level in human SW480 cells at 20 uM after 48 hrs by immunoblot analysis in presence of farnesyl pyrophosphate | 17472962 | |
| SW480 | Function assay | 20 uM | 48 hrs | Reversal of decrease in isoprenylated Ras level in human SW480 cells at 20 uM after 48 hrs by immunoblot analysis in presence of geranylgeranyl pyrophosphate | 17472962 | |
| SW480 | Function assay | 20 uM | Inhibition of FBS-stimulated increase in Ras protein expression in human SW480 cells assessed as GTP-bound protein at 20 uM by immunoblot analysis | 17472962 | ||
| SW480 | Function assay | 20 uM | Reversal of inhibition of FBS-stimulated increase in Ras protein expression in human SW480 cells assessed as GTP-bound protein at 20 uM by immunoblot analysis | 17472962 | ||
| SW480 | Function assay | 20 uM | Inhibition of FBS-stimulated increase in Akt phosphorylation in human SW480 cells at 20 uM by immunoblot analysis | 17472962 | ||
| LS180 | Growth inhibition assay | 72 hrs | Blockade of growth inhibition of human LS180 cells after 72 hrs by MTS method | 17472962 | ||
| K562 | Function assay | 48 hrs | Inhibition of GGTase1 in human K562 cells assessed as reduction of Rap1a protein geranylgeranylation after 48 hrs by Western blotting | 20832326 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells after 72 hrs by MTT assay, IC50=11.4μM. | 23570542 | ||
| A549 | Function assay | 5 mins | Inhibition of HMG-CoA reductase in human A549 cells after 5 mins by spectrophotometric analysis, IC50=19.8μM. | 23570542 | ||
| HS68 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HS68 cells after 72 hrs by MTT assay, IC50=23.2μM. | 23570542 | ||
| MEF | Cytotoxicity assay | 72 hrs | Cytotoxicity against mouse MEF cells after 72 hrs by MTT assay, IC50=35μM. | 23570542 | ||
| MDA-MB-231 | Function assay | 1 to 10 uM | 24 hrs | Induction of p21 expression in human PR, ER, HER2-negative human MDA-MB-231 cells at 1 to 10 uM after 24 hrs by western blot analysis | 24556504 | |
| MDA-MB-361 | Growth inhibition assay | 48 hrs | Growth inhibition of ER-positive, HER2-positive human MDA-MB-361 cells after 48 hrs by WST-1 assay | 24556504 | ||
| MDA-MB-468 | Growth inhibition assay | 48 hrs | Total growth inhibition of PR, ER, HER2-negative human MDA-MB-468 cells after 48 hrs by WST-1 assay | 24556504 | ||
| AU565 | Growth inhibition assay | 48 hrs | Growth inhibition of ER-negative, HER2-positive human AU565 cells after 48 hrs by WST-1 assay | 24556504 | ||
| MCF7 | Growth inhibition assay | 48 hrs | Total growth inhibition of ER-positive, HER2-negative human MCF7 cells after 48 hrs by WST-1 assay | 24556504 | ||
| MDA-MB-231 | Growth inhibition assay | >10 uM | 48 hrs | Total growth inhibition of PR, ER, HER2-negative human MDA-MB-231 cells at >10 uM after 48 hrs by WST-1 assay | 24556504 | |
| RPMI-8226 | Function assay | 10 uM | 48 hrs | Inhibition of HMG-coA reductase in human RPMI-8226 cells assessed as disruption of Rap1a geranylgeranylation at 10 uM after 48 hrs by western blot analysis | 24726306 | |
| HepG2 | Function assay | 1 uM | 6 hrs | Lipid-lowering effect in human HepG2 cells assessed as reduction in oleic acid-induced lipid accumulation at 1 uM after 6 hrs by oil-red O staining based spectrophotometry | 25304895 | |
| HepG2 | Function assay | 10 uM | 24 hrs | Lipid-lowering effect in human HepG2 cells assessed as reduction in oleic acid-induced total cholesterol accumulation at 10 uM after 24 hrs by oil-red O staining based spectrophotometry | 25304895 | |
| HepG2 | Function assay | 10 uM | 24 hrs | Lipid-lowering effect in human HepG2 cells assessed as reduction in oleic acid-induced triglyceride accumulation at 10 uM after 24 hrs by oil-red O staining based spectrophotometry | 25304895 | |
| RPMI8226 | Apoptosis assay | 20 uM | 48 hrs | Induction of apoptosis in human RPMI8226 cells assessed as increase in PARP cleavage at 20 uM incubated for 48 hrs by immunoblot method | 25935643 | |
| RPMI8226 | Apoptosis assay | 20 uM | 48 hrs | Induction of apoptosis in human RPMI8226 cells assessed as increase in caspase-3 cleavage at 20 uM incubated for 48 hrs by immunoblot method | 25935643 | |
| HepG2 | Function assay | 6 hrs | Lipid lowering activity in human HepG2 cells assessed as decrease in oleic acid elicited lipid accumulation after 6 hrs by oil-red O staining method, IC50=8.3μM. | 26169125 | ||
| A549 | Antiviral assay | 48 hrs | Antiviral activity against Dengue virus 2 NGC infected in human A549 cells assessed as reduction in virus replication after 48 hrs by renilla luciferase reporter gene assay, EC50=1.82μM. | 26771861 | ||
| Neuro2a | Function assay | 1 uM | Inhibition of HMGCoA reductase in Dhcr7-deficient mouse Neuro2a cells assessed as decrease in 7-DHC levels at 1 uM by LC-MS/GC-MS analysis | 26789657 | ||
| Vero | Cytotoxicity assay | Cytotoxicity against African green monkey Vero cells, IC50=2.2μM. | 27228159 | |||
| KB | Cytotoxicity assay | Cytotoxicity against human KB cells by resazurin microplate assay, IC50=15.6μM. | 27228159 | |||
| PC3 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human PC3 cells assessed as growth inhibition after 48 hrs by SRB assay, IC50=5.4μM. | 27756564 | ||
| MDA-MB-231 | Function assay | 30 uM | 24 hrs | Induction of reactive oxygen species in human MDA-MB-231 cells at 30 uM after 24 hrs by DCFH-DA probe-based flow cytometric method | 27756564 | |
| MDA-MB-231 | Function assay | 10 uM | 6 to 24 hrs | Induction of CHK1/2 phosphorylation in human MDA-MB-231 cells assessed as increase in p53 phosphorylation at 10 uM after 6 to 24 hrs by Western blot method | 27756564 | |
| MDA-MB-231 | Function assay | 10 uM | 6 to 24 hrs | Induction of ATM phosphorylation in human MDA-MB-231 cells at 10 uM after 6 to 24 hrs by Western blot method | 27756564 | |
| DAOY | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for DAOY cells | 29435139 | |||
| SJ-GBM2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SJ-GBM2 cells | 29435139 | |||
| Saos-2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Saos-2 cells | 29435139 | |||
| SK-N-SH | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-SH cells | 29435139 | |||
| BT-12 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-12 cells | 29435139 | |||
| Rh18 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh18 cells | 29435139 | |||
| OHS-50 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for OHS-50 cells | 29435139 | |||
| MG 63 (6-TG R) | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for MG 63 (6-TG R) cells | 29435139 | |||
| RPMI8226 | Function assay | 0.5 uM | 48 hrs | Inhibition of FTase in human RPMI8226 cells assessed as disruption of H-ras farnesylation at 0.5 uM after 48 hrs by immunoblot analysis | 31699606 | |
| RPMI8226 | Function assay | 0.5 uM | 48 hrs | Inhibition of GGtase-1 in human RPMI8226 cells assessed as disruption of Rap1a geranylgeranylation at 0.5 uM after 48 hrs by immunoblot analysis | 31699606 | |
| A549 | Function assay | Reductase Activity Assay: The HMGR activity was performed using HMG-CoA reductase assay kit from Sigma-Aldrich with the human recombinant protein or 100 μg total cell lysates from A549 cells. Lovastatin was used as a positive control, IC50=0.0295μM. | ChEMBL | |||
| HepG2 | Function assay | Compound was evaluated for inhibitory activity against HMG-CoA reductase in HepG2 cells, IC50=0.039μM. | ChEMBL | |||
| Click to View More Cell Line Experimental Data | ||||||
Biological Activity
| Description | Lovastatin is an inhibitor of HMG-CoA reductase with IC50 of 3.4 nM in a cell-free assay, used for lowering cholesterol (hypolipidemic agent). Lovastatin triggers autophagy. | ||
|---|---|---|---|
| Targets |
|
| In vitro | ||||
| In vitro | Lovastatin inhibits LPS- and cytokine-mediated production of NO and expression of iNOS in rat primary astrocytes. Lovastatin inhibits LPS-induced expression of TNF-alpha, IL-1beta, and IL-6 in rat primary astrocytes, microglia, and macrophages. [1] Lovastatin results in over 95% inhibition of DNA synthesis as measured by incorporation of [3H]thymidine into DNA. Lovastatin synchronizes cells in the G1 and not in the G0 phase of the cell cycle. Lovastatin has a similar growth-inhibitory activity against ras-dependent as well as ras-independent cell lines. [2] Lovastatin produces a profound reduction of apolipoprotein-B-containing lipoproteins, especially LDL cholesterol and, to a lesser extent, plasma triglyc- erides, and a small increase in HDL cholesterol. [3] Lovastatin arrests cells by inhibiting the proteasome, which results in the accumulation of p21 and p27, leading to G1 arrest. Lovastatin is an inhibitor of hydroxymethyl glutaryl (HMG)-CoA reductase, the rate-limiting enzyme in cholesterol synthesis. Lovastatin can be used to arrest cultured cells in the G1 phase of the cell cycle, resulting in the stabilization of the cyclin-dependent kinase inhibitors (CKIs) p21 and p27. [4] Lovastatin (2-10 mM) arrests cells in G1 and also prolonged--or arrested a minor fraction of cells in--the G2 phase of the cell cycle in human bladder carcinoma T24 cell line expressing activated p21ras. Lovastatin (50 mM) is cytotoxic in human bladder carcinoma T24 cell line expressing activated p21ras. [5] |
|||
|---|---|---|---|---|
| Cell Research | Cell lines | Astrocytes | ||
| Concentrations | 10 μM | |||
| Incubation Time | 8 h | |||
| Method | Cells preincubated in serum-free media with 10 µM lovastatin or 5 mM NaPA, or a combination of 2 µM lovastatin and 2 mM NaPA for 8 h received 1 µg/ml of LPS. |
|||
| Experimental Result Images | Methods | Biomarkers | Images | PMID |
| Western blot | p-AKT / AKT / p-GSK3β / GSK3β / p-β-catenin / β-catenin / TAZ HMGR |
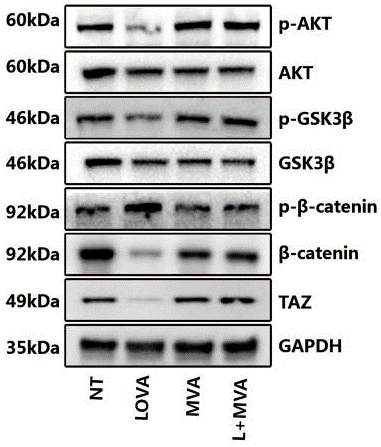
|
30975976 | |
| Immunofluorescence | β-catenin |
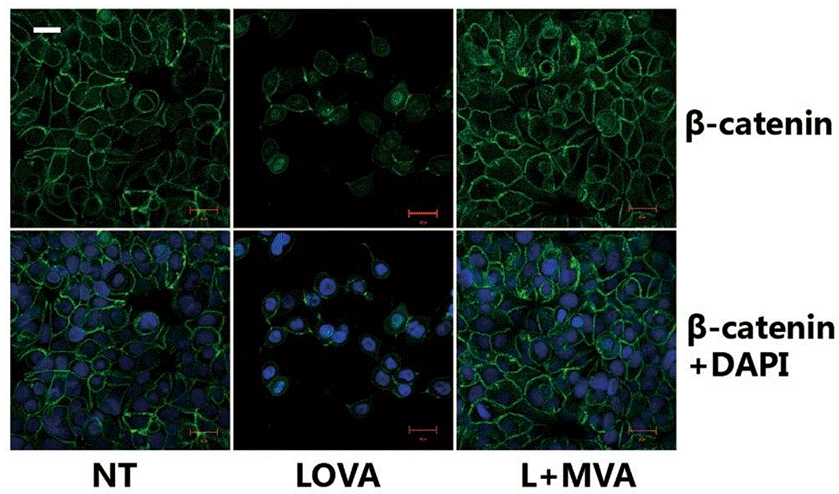
|
30975976 | |
| Growth inhibition assay | Cell viability |
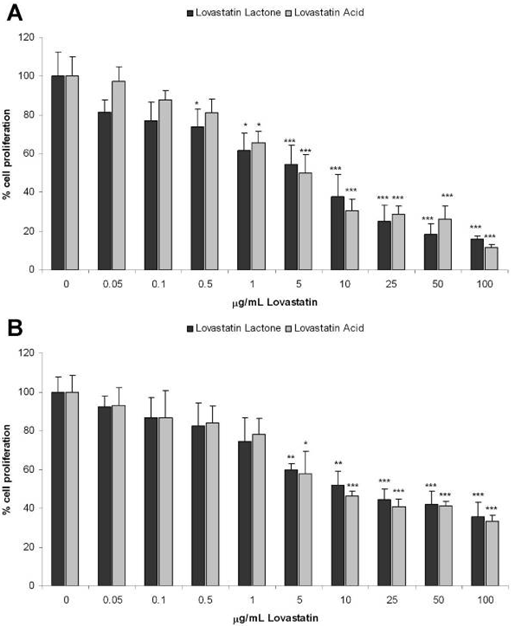
|
20205716 | |
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT01478828 | Terminated | Prostate Cancer |
Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins|Patrick C Walsh Prostate Cancer Research Fund |
July 13 2012 | Not Applicable |
| NCT01527669 | Completed | Healthy Subjects |
National Taiwan University Hospital|National Science Council Taiwan |
February 2012 | Phase 4 |
| NCT01385020 | Completed | Healthy Subjects |
National Taiwan University Hospital|National Science Council Taiwan |
July 2011 | Phase 4 |
| NCT00700921 | Completed | Chronic Obstructive Pulmonary Disease (COPD) |
National Jewish Health|National Heart Lung and Blood Institute (NHLBI) |
April 2008 | Phase 2 |
Chemical Information & Solubility
| Molecular Weight | 404.54 | Formula | C24H36O5 |
| CAS No. | 75330-75-5 | SDF | Download Lovastatin SDF |
| Smiles | CCC(C)C(=O)OC1CC(C=C2C1C(C(C=C2)C)CCC3CC(CC(=O)O3)O)C | ||
| Storage (From the date of receipt) | |||
|
In vitro |
DMSO : 80 mg/mL ( (197.75 mM); Moisture-absorbing DMSO reduces solubility. Please use fresh DMSO.) Ethanol : 35 mg/mL Water : Insoluble |
Molecular Weight Calculator |
|
In vivo Add solvents to the product individually and in order. |
In vivo Formulation Calculator |
||||
Preparing Stock Solutions
Molarity Calculator
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Tech Support
Answers to questions you may have can be found in the inhibitor handling instructions. Topics include how to prepare stock solutions, how to store inhibitors, and issues that need special attention for cell-based assays and animal experiments.
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
* Indicates a Required Field
Tags: buy Lovastatin | Lovastatin supplier | purchase Lovastatin | Lovastatin cost | Lovastatin manufacturer | order Lovastatin | Lovastatin distributor