- Bioactive Compounds
- By Signaling Pathways
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- Compound Libraries
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-Ⅱ
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Plant Extract Library
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at [email protected] to customize your library.
You could select:
- Antibodies
- Bioreagents
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- Protein Assay
- Protein A/G Magnetic Beads for IP
- Anti-Flag magnetic beads
- Anti-Flag Affinity Gel
- Anti-Myc magnetic beads
- Anti-HA magnetic beads
- Poly FLAG Peptide lyophilized powder
- Protease Inhibitor Cocktail
- Protease Inhibitor Cocktail (EDTA-Free, 100X in DMSO)
- Phosphatase Inhibitor Cocktail (2 Tubes, 100X)
- Cell Biology
- Cell Counting Kit-8 (CCK-8)
- Animal Experiment
- Mouse Direct PCR Kit (For Genotyping)
- New Products
- Contact Us
Celastrol
Synonyms: NSC 70931, Tripterine
Celastrol is a potent proteasome inhibitor for the chymotrypsin-like activity of a purified 20S proteasome with IC50 of 2.5 μM. Celastrol induces apoptosis and autophagy via the ROS/JNK signaling pathway. Celastrol inhibits dopaminergic neuronal death of Parkinson's disease through activating mitophagy.
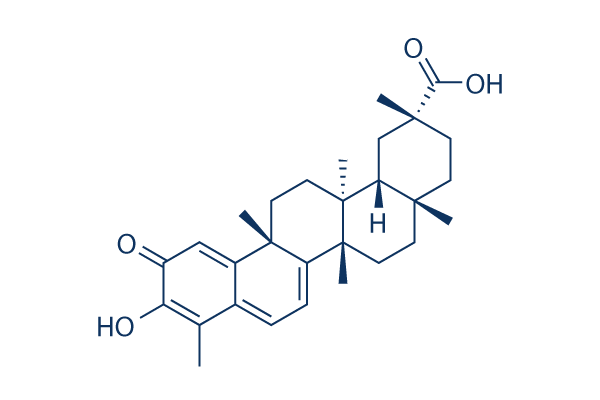
Celastrol Chemical Structure
CAS: 34157-83-0
Selleck's Celastrol has been cited by 19 Publications
2 Customer Reviews
Purity & Quality Control
Batch:
Purity:
99.56%
99.56
Celastrol Related Products
| Related Targets | 20S proteasome | Click to Expand |
|---|---|---|
| Related Products | MG132 Carfilzomib (PR-171) Ixazomib (MLN2238) Epoxomicin (BU-4061T) Oprozomib ONX-0914 (PR-957) Delanzomib Ixazomib Citrate (MLN9708) VR23 Marizomib (Salinosporamide A) Ixazomib Citrate (MLN9708) Analogue PI-1840 | Click to Expand |
| Related Compound Libraries | FDA-approved Drug Library Natural Product Library Bioactive Compound Library-I Protease Inhibitor Library Ubiquitination Compound Library | Click to Expand |
Signaling Pathway
Choose Selective Proteasome Inhibitors
Cell Data
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| RAW264.7 | Antiinflammatory assay | 24 hrs | Antiinflammatory activity against mouse RAW264.7 cells assessed as inhibition of LPS-induced NO production treated 3 hrs before LPS challenge assessed after 24 hrs by Griess reagent assay, IC50 = 0.23 μM. | 11809076 | ||
| RAW264.7 | Antiinflammatory assay | 24 hrs | Antiinflammatory activity against mouse RAW264.7 cells assessed as inhibition of LPS-induced NF-kappaB activation treated 3 hrs before LPS challenge assessed after 24 hrs by SEAP reporter gene assay, IC50 = 0.27 μM. | 11809076 | ||
| RPMI8226 | Growth inhibition assay | Growth inhibition of human RPMI8226 cells, IC50 = 3 μM. | 18164197 | |||
| HeLa | Function assay | Inhibition of TNF-alpha-induced NF-kappaB activation in human HeLa cells by SEAP reporter gene assay, IC50 = 0.15 μM. | 18841906 | |||
| RAW264.7 | Function assay | Inhibition of LPS-induced NF-kappaB activation in mouse RAW264.7 cells by SEAP reporter gene assay, IC50 = 0.3 μM. | 18841906 | |||
| SH-SY5Y | Function assay | Neuroprotection against beta-amyloid peptide 1-42-induced toxicity in human SH-SY5Y cells assessed as lactate dehydrogenase release, EC50 = 0.03764 μM. | 19138859 | |||
| HeLa | Function assay | 2 hrs | Amplification of HSF1 transcriptional activity in human heat shock-induced HeLa cells assessed as granule formation treated 1 hr before heat shock challenge measured after 2 hrs without recovery time by immunocytochemical staining, EC50 = 1.1 μM. | 19502057 | ||
| SK-N-SH | Cytotoxicity assay | 72 hrs | Cytotoxicity against human SK-N-SH cells after 72 hrs by MTS assay, CC50 = 1.6 μM. | 19502057 | ||
| HeLa | Function assay | 3 hrs | Amplification of HSF1 transcriptional activity in human HeLa cells assessed as granule formation pretreated for 3 hrs by immunocytochemical staining, EC50 = 2.6 μM. | 19502057 | ||
| HeLa | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HeLa cells after 72 hrs by MTS assay, CC50 = 3 μM. | 19502057 | ||
| HeLa | Function assay | Inhibition of NF-kappaB activity in human HeLa cells by SEAP reporter assay, IC50 = 0.15 μM. | 20469887 | |||
| LNCAP | Antitumor assay | Antitumor activity against human LNCAP cells, IC50 = 2.5 μM. | 20627556 | |||
| PC12 | Cytoprotective assay | Cytoprotective activity against t-BPH-induced cell damage in rat PC12 cells assessed as cell viability, IC50 = 3.15 μM. | 20627556 | |||
| PC12 | Cytoprotective assay | 1.6 uM | Cytoprotective activity against t-BPH-induced cell damage in rat PC12 cells assessed as cell viability at 1.6 uM | 20627556 | ||
| THP1 | Apoptosis assay | 24 hrs | Induction of apoptosis in TRAIL-resistant human THP1 cells after 24 hrs by annexin-V staining, EC50 = 15 μM. | 20864342 | ||
| 293T | Function assay | 30 mins | Inhibition of LPS-induced NF-kappaB activation in human 293T cells incubated for 30 mins followed by treated with LPS for 6 hrs by dual-luciferase reporter assay, IC50 = 0.17 μM. | 21393004 | ||
| 293T | Cytotoxicity assay | Cytotoxicity against human 293T cells assessed as cell viability by dual-luciferase reporter assay, IC50 = 0.67 μM. | 21393004 | |||
| RAW264 | Function assay | 24 hrs | Inhibition of LPS-induced NO production in mouse RAW264 cells after 24 hrs | 21393004 | ||
| NCI-H460 | Cytotoxicity assay | Cytotoxicity against human NCI-H460 cells after overnight incubation by MTT assay, IC50 = 12.3 μM. | 21942765 | |||
| RAW264.7 | Antiinflammatory assay | 24 hrs | Antiinflammatory activity in mouse RAW264.7 cells assessed as inhibition of LPS-induced NO production after 24 hrs by Griess reagent method, IC50 = 1 μM. | 21978676 | ||
| RAW264.7 | Antiinflammatory assay | 24 hrs | Antiinflammatory activity in mouse RAW264.7 cells assessed as inhibition of LPS-induced nitric oxide production after 24 hrs by griess assay, IC50 = 1 μM. | 22024033 | ||
| RAW264.7 | Function assay | 18 hrs | Inhibition of LPS-stimulated NFkappaB activation transfected in mouse RAW264.7 cells after 18 hrs by luciferase reporter gene assay, IC50 = 0.2 μM. | 22705020 | ||
| spleen adherent cells | Antiarthritic assay | 200 ug | Antiarthritic effect in Lewis rat adjuvant-induced arthritis model spleen adherent cells restimulated with sonicated Mtb post isolation assessed as reduction in CCR2 expression at 200 ug, ip starting from arthritis onset and continued uninterrupted until | 22854193 | ||
| spleen adherent cells | Antiarthritic assay | 200 ug | Antiarthritic effect in Lewis rat adjuvant-induced arthritis model spleen adherent cells restimulated with sonicated Mtb post isolation assessed as reduction in GRO/KC production at 200 ug, ip starting from arthritis onset and continued uninterrupted unti | 22854193 | ||
| spleen adherent cells | Antiarthritic assay | 200 ug | Antiarthritic effect in Lewis rat adjuvant-induced arthritis model spleen adherent cells restimulated with sonicated Mtb post isolation assessed as reduction in MCP1 production at 200 ug, ip starting from arthritis onset and continued uninterrupted until | 22854193 | ||
| spleen adherent cells | Antiarthritic assay | 200 ug | Antiarthritic effect in Lewis rat adjuvant-induced arthritis model spleen adherent cells restimulated with sonicated Mtb post isolation assessed as reduction in RANTES production at 200 ug, ip starting from arthritis onset and continued uninterrupted unti | 22854193 | ||
| spleen adherent cells | Antiarthritic assay | 200 ug | Antiarthritic effect in Lewis rat adjuvant-induced arthritis model spleen adherent cells restimulated with sonicated Mtb post isolation assessed as reduction in TNFalpha production at 200 ug, ip starting from arthritis onset and continued uninterrupted un | 22854193 | ||
| spleen adherent cells | Antiarthritic assay | 200 ug | Antiarthritic effect in Lewis rat adjuvant-induced arthritis model spleen adherent cells restimulated with sonicated Mtb post isolation assessed as reduction in CCR3 expression at 200 ug, ip starting from arthritis onset and continued uninterrupted until | 22854193 | ||
| spleen adherent cells | Antiarthritic assay | 200 ug | Antiarthritic effect in Lewis rat adjuvant-induced arthritis model spleen adherent cells restimulated with sonicated Mtb post isolation assessed as reduction in CCR6 expression at 200 ug, ip starting from arthritis onset and continued uninterrupted until | 22854193 | ||
| spleen adherent cells | Antiarthritic assay | 200 ug | Antiarthritic effect in Lewis rat adjuvant-induced arthritis model spleen adherent cells restimulated with sonicated Mtb post isolation assessed as reduction in CXCR1 expression at 200 ug, ip starting from arthritis onset and continued uninterrupted until | 22854193 | ||
| spleen adherent cells | Antiarthritic assay | 200 ug | Antiarthritic effect in Lewis rat adjuvant-induced arthritis model spleen adherent cells restimulated with sonicated Mtb post isolation assessed as reduction in CXCR2 expression at 200 ug, ip starting from arthritis onset and continued uninterrupted until | 22854193 | ||
| spleen adherent cells | Antiarthritic assay | 200 ug | Antiarthritic effect in Lewis rat adjuvant-induced arthritis model spleen adherent cells restimulated with sonicated Mtb post isolation assessed as reduction in CXCR4 expression at 200 ug, ip starting from arthritis onset and continued uninterrupted until | 22854193 | ||
| spleen adherent cells | Function assay | 200 ug | Increase in CCR1 expression in Lewis rat adjuvant-induced arthritis model spleen adherent cells restimulated with sonicated Mtb post isolation at 200 ug, ip starting from arthritis onset and continued uninterrupted until study end by qPCR relative baselin | 22854193 | ||
| spleen adherent cells | Antiarthritic assay | 200 ug | Antiarthritic effect in Lewis rat adjuvant-induced arthritis model spleen adherent cells restimulated with sonicated Mtb post isolation assessed as reduction in IL-1beta production at 200 ug, ip starting from arthritis onset and continued uninterrupted un | 22854193 | ||
| spleen adherent cells | Antiarthritic assay | 200 ug | Antiarthritic effect in Lewis rat adjuvant-induced arthritis model spleen adherent cells restimulated with sonicated Mtb post isolation assessed as reduction in CCR5 expression at 200 ug, ip starting from arthritis onset and continued uninterrupted until | 22854193 | ||
| spleen adherent cells | Function assay | 24 hrs | Increase in CCR1 protein surface expression in sonicated Mtb-stimulated spleen adherent cells isolated from Lewis rat adjuvant-induced arthritis model pre-incubated with 0.1 to 0.3 uM compound before stimulation with sonicated Mtb for 24 hrs by flow cytom | 22854193 | ||
| RAW264.7 | Antiinflammatory assay | 24 hrs | Antiinflammatory activity in mouse RAW264.7 cells assessed as inhibition of LPS-induced NO production after 24 hrs by Griess method, IC50 = 1 μM. | 23127886 | ||
| RAW264.7 | Antiinflammatory assay | 24 hrs | Antiinflammatory activity in mouse RAW264.7 cells assessed as inhibition of LPS-induced TNFalpha production incubated for 24 hrs by ELISA, IC50 = 0.8 μM. | 23234407 | ||
| RAW264.7 | Antiinflammatory assay | 24 hrs | Antiinflammatory activity in mouse RAW264.7 cells assessed as inhibition of LPS-induced nitric oxide generation incubated for 24 hrs, IC50 = 1 μM. | 23234407 | ||
| RAW264.7 | Antiinflammatory assay | Anti-inflammatory activity in mouse RAW264.7 cells assessed as inhibition of LPS-induced nitric oxide production by Griess reaction based method, IC50 = 1 μM. | 25637363 | |||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells assessed as growth inhibition after 72 hrs by MTS/PMS assay, IC50 = 0.153 μM. | 25756299 | ||
| MCF7 | Function assay | 24 hrs | Inhibition of HSP90 in human MCF7 cells assessed as pAkt degradation at 5 times IC50 after 24 hrs by Western blot analysis | 25756299 | ||
| MCF7 | Function assay | 24 hrs | Inhibition of HSP90 in human MCF7 cells assessed as Her2 degradation at 5 times IC50 after 24 hrs by Western blot analysis | 25756299 | ||
| MCF7 | Function assay | 24 hrs | Inhibition of HSP90 in human MCF7 cells assessed as Cdk6 degradation at 5 times IC50 after 24 hrs by Western blot analysis | 25756299 | ||
| MCF7 | Function assay | 24 hrs | Inhibition of HSP90 in human MCF7 cells assessed as Raf degradation at 5 times IC50 after 24 hrs by Western blot analysis | 25756299 | ||
| MCF7 | Function assay | 24 hrs | Change in Cdc37 expression in human MCF7 cells at 5 times IC50 after 24 hrs by Western blot analysis | 25756299 | ||
| MCF7 | Function assay | 24 hrs | Change in p23 expression in human MCF7 cells at 5 times IC50 after 24 hrs by Western blot analysis | 25756299 | ||
| MCF7 | Function assay | 24 hrs | Inhibition of HSP90 in human MCF7 cells assessed as disruption of interaction with Cdc37 at 5 times IC50 after 24 hrs by co-immunoprecipitation assay | 25756299 | ||
| MCF7 | Function assay | 24 hrs | Inhibition of HSP90 in human MCF7 cells assessed as disruption of interaction with p23 at 5 times IC50 after 24 hrs by co-immunoprecipitation assay | 25756299 | ||
| SGC7901 | Anticancer assay | 48 hrs | Anticancer activity against human SGC7901 cells assessed as cell survival after 48 hrs by MTT assay, IC50 = 0.15 μM. | 25812966 | ||
| SMMC7721 | Anticancer assay | 48 hrs | Anticancer activity against human SMMC7721 cells assessed as cell survival after 48 hrs by MTT assay, IC50 = 0.58 μM. | 25812966 | ||
| MGC803 | Anticancer assay | 48 hrs | Anticancer activity against human MGC803 cells assessed as cell survival after 48 hrs by MTT assay, IC50 = 1.55 μM. | 25812966 | ||
| HepG2 | Anticancer assay | 48 hrs | Anticancer activity against human HepG2 cells assessed as cell survival after 48 hrs by MTT assay, IC50 = 4.01 μM. | 25812966 | ||
| A549 | Antiproliferative assay | 48 hrs | Antiproliferative activity against paclitaxel-resistant human A549 cells assessed as reduction in cell viability after 48 hrs by MTT assay, IC50 = 2.01 μM. | 27647369 | ||
| A549 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human A549 cells assessed as reduction in cell viability after 48 hrs by MTT assay, IC50 = 2.02 μM. | 27647369 | ||
| MCF7 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MCF7 cells assessed as reduction in cell viability after 48 hrs by MTT assay, IC50 = 2.13 μM. | 27647369 | ||
| PANC1 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human PANC1 cells assessed as reduction in cell viability after 48 hrs by MTT assay, IC50 = 2.48 μM. | 27647369 | ||
| LNCAP | Function assay | 5 uM | 24 hrs | Antagonist activity at AR in human LNCAP cells assessed as suppression of DHT-induced receptor transcriptional activity at 5 uM after 24 hrs by dual luciferase reporter gene assay | 27994731 | |
| A549 | Growth inhibition assay | 6 days | Growth inhibition of human A549 cells measured every 2 hrs over 6 days by live cell imaging based method, IC50 = 0.69 μM. | 28621943 | ||
| HEK293 | Function assay | 10 uM | 20 mins | Inhibition of Galphao interaction with GFP-fused RGS17 (unknown origin) deltaN mutant expressed in HEK293 cells assessed as increase in RGS17 deltaN mutant localization at cytoplasm at 10 uM up to 20 mins by confocal microscopic analysis | 28621943 | |
| HEK293 | Function assay | 100 uM | 5 mins | Inhibition of Galpha0 interaction with GFP-fused RGS17 (unknown origin) deltaN mutant expressed in HEK293 cells assessed as increase in RGS17 deltaN mutant localization at cytoplasm at 100 uM up to 5 mins by confocal microscopic analysis | 28621943 | |
| HEK293 | Function assay | 10 uM | 5 mins | Inhibition of Galphao interaction with GFP-fused RGS17 (unknown origin) deltaN mutant expressed in HEK293 cells assessed as increase in RGS17 deltaN mutant localization at cytoplasm at 10 uM up to 5 mins by confocal microscopic analysis | 28621943 | |
| MIAPaCa2 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MIAPaCa2 cells after 72 hrs by MTT assay, IC50 = 0.46 μM. | 28688281 | ||
| SKBR3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SKBR3 cells after 72 hrs by MTT assay, IC50 = 0.72 μM. | 28688281 | ||
| SKOV3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SKOV3 cells after 72 hrs by MTT assay, IC50 = 1.16 μM. | 28688281 | ||
| MDA-MB-231 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MDA-MB-231 cells after 72 hrs by MTT assay, IC50 = 1.32 μM. | 28688281 | ||
| A549 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human A549 cells after 72 hrs by MTT assay, IC50 = 1.56 μM. | 28688281 | ||
| BJ | Cytotoxicity assay | 72 hrs | Cytotoxicity against human BJ cells assessed as reduction in cell viability after 72 hrs by MTT assay, IC50 = 2.74 μM. | 28688281 | ||
| NCI-H460 | Function assay | 1 uM | 12 hrs | Activation of HSF1 in human NCI-H460 cells assessed as upregulation of HSP25 protein levels at 1 uM after 12 hrs by Western blot analysis | 28737916 | |
| NCI-H460 | Function assay | 1 uM | 12 hrs | Activation of HSF1 in human NCI-H460 cells assessed as upregulation of HSP70 protein levels at 1 uM after 12 hrs by Western blot analysis | 28737916 | |
| NCI-H1299 | Cytotoxicity assay | Cytotoxicity in human NCI-H1299 cells assessed as reduction in cell viability, IC50 = 1 μM. | 28754470 | |||
| BCP-ALL | Cytotoxicity assay | Cytotoxicity in human BCP-ALL cells assessed as reduction in cell viability, IC50 = 1 μM. | 28754470 | |||
| T-ALL | Cytotoxicity assay | Cytotoxicity in human T-ALL cells assessed as reduction in cell viability, IC50 = 1 μM. | 28754470 | |||
| Daudi | Cytotoxicity assay | Cytotoxicity in human Daudi cells assessed as reduction in cell viability, IC50 = 1 μM. | 28754470 | |||
| HL60 | Cytotoxicity assay | Cytotoxicity in human HL60 cells assessed as reduction in cell viability, IC50 = 1 μM. | 28754470 | |||
| TC32 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for TC32 cells | 29435139 | |||
| DAOY | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for DAOY cells | 29435139 | |||
| SJ-GBM2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SJ-GBM2 cells | 29435139 | |||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for A673 cells | 29435139 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-MC cells | 29435139 | |||
| BT-37 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-37 cells | 29435139 | |||
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB-EBc1 cells | 29435139 | |||
| Saos-2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Saos-2 cells | 29435139 | |||
| SK-N-SH | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-SH cells | 29435139 | |||
| NB1643 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB1643 cells | 29435139 | |||
| LAN-5 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for LAN-5 cells | 29435139 | |||
| BT-12 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-12 cells | 29435139 | |||
| Rh18 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh18 cells | 29435139 | |||
| OHS-50 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for OHS-50 cells | 29435139 | |||
| RD | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for RD cells | 29435139 | |||
| MG 63 (6-TG R) | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for MG 63 (6-TG R) cells | 29435139 | |||
| Rh30 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh30 cells | 29435139 | |||
| Rh41 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh41 cells | 29435139 | |||
| NB1643 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for NB1643 cells | 29435139 | |||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for A673 cells) | 29435139 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for SK-N-MC cells | 29435139 | |||
| BT-12 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for BT-12 cells | 29435139 | |||
| LAN-5 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for LAN-5 cells | 29435139 | |||
| DAOY | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for DAOY cells | 29435139 | |||
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for NB-EBc1 cells | 29435139 | |||
| SJ-GBM2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for SJ-GBM2 cells | 29435139 | |||
| BT-37 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for BT-37 cells | 29435139 | |||
| TC32 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for TC32 cells | 29435139 | |||
| MG 63 (6-TG R) | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for MG 63 (6-TG R) cells | 29435139 | |||
| fibroblast cells | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for control Hh wild type fibroblast cells | 29435139 | |||
| U-2 OS | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for U-2 OS cells | 29435139 | |||
| Rh41 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for Rh41 cells | 29435139 | |||
| RD | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for RD cells | 29435139 | |||
| Rh18 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for Rh18 cells | 29435139 | |||
| Rh30 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for Rh30 cells | 29435139 | |||
| Saos-2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for Saos-2 cells | 29435139 | |||
| OHS-50 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for OHS-50 cells | 29435139 | |||
| SK-N-SH | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for SK-N-SH cells | 29435139 | |||
| HCT116 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HCT116 cells after 48 hrs by MTT assay, IC50 = 5.26 μM. | 29486954 | ||
| HepG2 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human HepG2 cells after 48 hrs by MTT assay, IC50 = 6.17 μM. | 29486954 | ||
| A549 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human A549 cells after 48 hrs by MTT assay, IC50 = 6.59 μM. | 29486954 | ||
| MCF7 | Antiproliferative assay | 48 hrs | Antiproliferative activity against human MCF7 cells after 48 hrs by MTT assay, IC50 = 6.84 μM. | 29486954 | ||
| HEK293 | Cytotoxicity assay | Cytotoxicity against HEK293 cells (CO-ADD:MA_007); CC50 by cell viability assay in DMEM (10% FBS) media using TC plates, by Resazurin F(560/590), CC50 = 0.837 μM. | ChEMBL | |||
| Click to View More Cell Line Experimental Data | ||||||
Biological Activity
| Description | Celastrol is a potent proteasome inhibitor for the chymotrypsin-like activity of a purified 20S proteasome with IC50 of 2.5 μM. Celastrol induces apoptosis and autophagy via the ROS/JNK signaling pathway. Celastrol inhibits dopaminergic neuronal death of Parkinson's disease through activating mitophagy. | ||
|---|---|---|---|
| Features | A potent antioxidant and anti-inflammatory drug. | ||
| Targets |
|
| In vitro | ||||
| In vitro | Celastrol at 5 μM inhibits the chymotrypsin-like, PGPH-like, and trypsin-like activities of the purified 20S proteasome by 80%, 5%, and <1%, respectively, whereas at 10 μM, it inhibits these three proteasomal activities by ∼90%, 15%, and <1%, respectively. Celastrol significantly inhibits the proteasomal chymotrypsin activity in PC-3 cells in a concentration-dependent manner. Celastrol at 2.5 μM to 5 μM induces caspase-3 activity by 4.7-fold to 5.5-fold in PC-3 cells. Celastrol (5 μM) treated cells, the levels of the proteasome target proteins, IκB-α and Bax, are increased after 1 hour and further increased to its peak for 4 hours to 12 hours. Celastrol (2.5 μM) treatment induces proteasome inhibition by 40%, as shown by the decreased levels of chymotrypsin-like activity and increased accumulation of ubiquitinated proteins in LNCaP cells. Celastrol (2.5 μM) induces apoptosis in the Celastrol-treated LNCaP cells, as shown by increased levels of caspase-3 activity (up to 3.5-fold), PARP cleavage, and apoptotic morphology. [1] Celastrol (300 nM) is found to suppress LPS-induced production of TNF-alpha and IL-1beta by human monocytes and macrophages. Celastrol (100 nM) also decreases LPS-induced expression of class II MHC molecules by microglia. Celastrol strongly inhibits LPS and IFN-y-induced NO production with IC50 of 200 nM in macrophage lineage cells. Celastrol strongly inhibits TNF-α and IFN-γ-induced NO production with IC50 of 200 nM in endothelial cells. [2] Celastrol (2.5 μM) potentiates the apoptosis induced by TNF and chemotherapeutic agents and inhibits invasion, both regulated by NF-kappaB activation, in KBM-5 cells. Celastrol (2.5 μM) suppresses the expression of TNF induced the expression of gene products involved in antiapoptosis (IAP1, IAP2, Bcl-2, Bcl-XL, c-FLIP, and survivin), proliferation (cyclin D1 and COX-2), invasion (MMP-9), and angiogenesis (VEGF) in KBM-5 cells. Celastrol (5 μM) is found to inhibit the TNF-induced activation of IkappaBalpha kinase, IkappaBalpha phosphorylation, IkappaBalpha degradation, p65 nuclear translocation and phosphorylation, and NF-kappaB-mediated reporter gene expression. [3] Celastrol inhibits proliferating of RPMI 8226, KATOIII, UM-SCC1, U251MG and MDA-MB-231 cells with IC50 of 0.52 μM, 0.54 μM, 0.76 μM, 0.69 μM and 0.67 μM, respectively. Celastrol (1 μM) inhibits growth of RPMI 8226 with a decrease in the levels of cyclin D1 and cyclin E, but concomitant increase in the levels of p21 and p27. Celastrol induces apoptosis in RPMI-8226 cells indicated by the activation of caspase-8, bid cleavage, caspase-9 activation, caspase-3 activation, PARP cleavage and through the down regulation of anti-apoptototic proteins. Celastrol (1 μM) suppresses Akt pathway and activates JNK kinase in RPMI-8226 cells. [4] | |||
|---|---|---|---|---|
| Kinase Assay | Inhibition of purified 20S proteasome activity | |||
| A purified rabbit 20S proteasome (0.1 μg) is incubated with 40 μM of various fluorogenic peptide substrates in 100 μL assay buffer (20 mM Tris-HCl (pH 7.5)), in the presence of Celastrol at different concentrations or in the solvent DMSO for 2 hours at 37 ℃, followed by measurement of inhibition of each proteasomal activity. | ||||
| Cell Research | Cell lines | RPMI 8226, KATOIII, UM-SCC1, U251MG and MDA-MB-231 cells | ||
| Concentrations | ~5 μM | |||
| Incubation Time | 2 hours | |||
| Method | The anti-proliferative effect of celastrol on various human tumor cell lines is determined by the MTT uptake method. Briefly, 5×103 cells are incubated with Celastrol in triplicate in a 96-well plate at 37 ℃. MTT solution is then added to each well. After a 2 hours incubation at 37 ℃, extraction buffer (20% SDS, 50% dimethylformamide) is added, cells are incubated overnight at 37 ℃, and the optical density is then measured at 570 nm using a Tecan plate reader. | |||
| Experimental Result Images | Methods | Biomarkers | Images | PMID |
| Western blot | HIF-1α Akt / p-Akt / p-p70S6K PARP / p53 / p21 / cIAP1 / Bcl-xl / Bcl-2 IκBα / p-IKKα/β iNOS / COX-2 / Arg-1 Chk2 / p-Chk2 / Cyclin B1 / Cdc25c / p-Cdc25c |
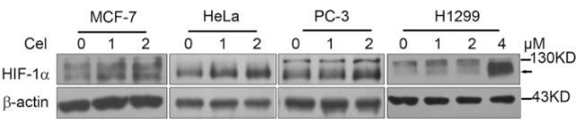
|
25383959 | |
| Immunofluorescence | p21 / Nur77 |
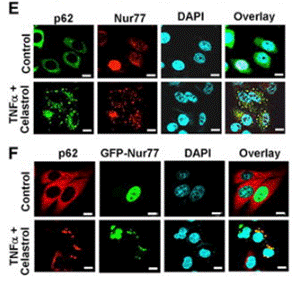
|
28388439 | |
| Growth inhibition assay | Cell viability |
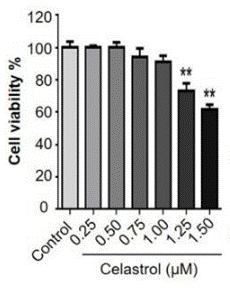
|
29040966 | |
| In Vivo | ||
| In vivo | Celastrol (3 mg/kg) results in significant inhibition (up to 70%) of tumor growth in male nude mice bearing PC-3 tumors, associated with increased p27 levels and Bax level. Celastrol (3 mg/kg) results more apoptotic tumor cells with the appearance of various PARP cleavage fragments in tumor of male nude mice bearing PC-3 tumors. Celastrol (3 mg/kg) causes 35% of tumor inhibition, associated with decreased proteasome activity and decreased expression of AR protein in nude mice bearing C4-2B tumors. [1] Celastrol (3 mg/kg) is found to suppress strongly joint swelling and other manifestations of adjuvant arthritis in mice. Celastrol (0.2 mg/kg) significantly improves the performance in memory, learning and psychomotor activity tests in rats. [2] | |
|---|---|---|
| Animal Research | Animal Models | nude mice bearing C4-2B tumors |
| Dosages | 3 mg/kg | |
| Administration | Intraperitoneal injection | |
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05494112 | Recruiting | Safety |
Legend Labz Inc. |
May 25 2022 | Not Applicable |
| NCT05413226 | Recruiting | Safety Issues |
Legend Labz Inc. |
September 28 2021 | Not Applicable |
Chemical Information & Solubility
| Molecular Weight | 450.61 | Formula | C29H38O4 |
| CAS No. | 34157-83-0 | SDF | Download Celastrol SDF |
| Smiles | CC1=C(C(=O)C=C2C1=CC=C3C2(CCC4(C3(CCC5(C4CC(CC5)(C)C(=O)O)C)C)C)C)O | ||
| Storage (From the date of receipt) | |||
|
In vitro |
DMSO : 90 mg/mL ( (199.72 mM); Moisture-absorbing DMSO reduces solubility. Please use fresh DMSO.) Water : Insoluble Ethanol : Insoluble |
Molecular Weight Calculator |
|
In vivo Add solvents to the product individually and in order. |
In vivo Formulation Calculator |
||||
Preparing Stock Solutions
Molarity Calculator
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Tech Support
Answers to questions you may have can be found in the inhibitor handling instructions. Topics include how to prepare stock solutions, how to store inhibitors, and issues that need special attention for cell-based assays and animal experiments.
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
* Indicates a Required Field
Tags: buy Celastrol | Celastrol supplier | purchase Celastrol | Celastrol cost | Celastrol manufacturer | order Celastrol | Celastrol distributor